- Department of Neurosurgery, Yokohama Sakae Kyosai Hospital, Yokohama, Kanagawa, Japan.
- Department of Neurology, Yokohama Sakae Kyosai Hospital, Yokohama, Kanagawa, Japan.
Correspondence Address:
Takeshi Kidoguchi, Department of Neurosurgery, Yokohama Sakae Kyosai Hospital, Yokohama, Kanagawa, Japan.
DOI:10.25259/SNI_184_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takeshi Kidoguchi1, Issei Fukui1, Hiroyuki Abe1, Kentaro Mori1, Akira Tamase1, Ryotaro Yamashita2, Mutsuki Takeda2, Tatsu Nakano2, Motohiro Nomura1. Carotid artery stenting for spontaneous internal carotid artery dissection presenting with hypoglossal nerve palsy: A case report. 27-May-2022;13:225
How to cite this URL: Takeshi Kidoguchi1, Issei Fukui1, Hiroyuki Abe1, Kentaro Mori1, Akira Tamase1, Ryotaro Yamashita2, Mutsuki Takeda2, Tatsu Nakano2, Motohiro Nomura1. Carotid artery stenting for spontaneous internal carotid artery dissection presenting with hypoglossal nerve palsy: A case report. 27-May-2022;13:225. Available from: https://surgicalneurologyint.com/surgicalint-articles/11618/
Abstract
Background: Some studies reported cases of internal carotid artery (ICA) dissection (ICAD) that was treated by carotid artery stenting (CAS). Symptoms of ICAD resulting from the lower cranial nerve palsy are rare and the treatment strategy is not clearly defined. We report a patient with ICAD showing hypoglossal nerve palsy alone that was treated by CAS.
Case Description: A 47-year-old man presented with headache, dysphagia, dysarthria, and tongue deviation to the left. He had no history of trauma nor any other significant medical history. Axial T2-CUBE MRI and MRA showed dissection of the left ICA accompanied with a false lumen. These findings indicated that direct compression by the false lumen was the cause of hypoglossal nerve palsy. Although medical treatment was continued, symptoms were not improved. Therefore, CAS was performed to thrombose the false lumen and decompress the hypoglossal nerve. His symptoms gradually improved after CAS and angiography performed at month 6 showed well-dilated ICA and disappearance of false lumen.
Conclusion: CAS may be an effective treatment for the lower cranial nerve palsy caused by compression by a false lumen of ICAD.
Keywords: Carotid artery stenting, Hypoglossal nerve palsy, Internal carotid artery dissection, Lower cranial nerve palsy
INTRODUCTION
Carotid artery stenting (CAS) has become the definite treatment for internal carotid artery (ICA) stenosis. Moreover, some studies reported cases of ICA dissection (ICAD) that was treated by CAS. CAS is performed for patients with ICAD whose symptoms are not controlled by antithrombotic drugs or who are at high risk of stroke.[
CASE PRESENTATION
A 47-year-old man was initially diagnosed as dysphagia. On the next day, he developed dysarthria and tongue deviation to the left, and he visited our hospital. He had no history of trauma nor any other significant medical history. Blood pressure was 184/126 mmHg. Neurological examinations revealed leftward deviation of the tongue on protrusion and dysarthria, suggesting a left hypoglossal nerve palsy. There were no other neurological deficits. Diffusion-weighted magnetic resonance images (MRI) did not demonstrate any lesions that could cause left hypoglossal nerve palsy [
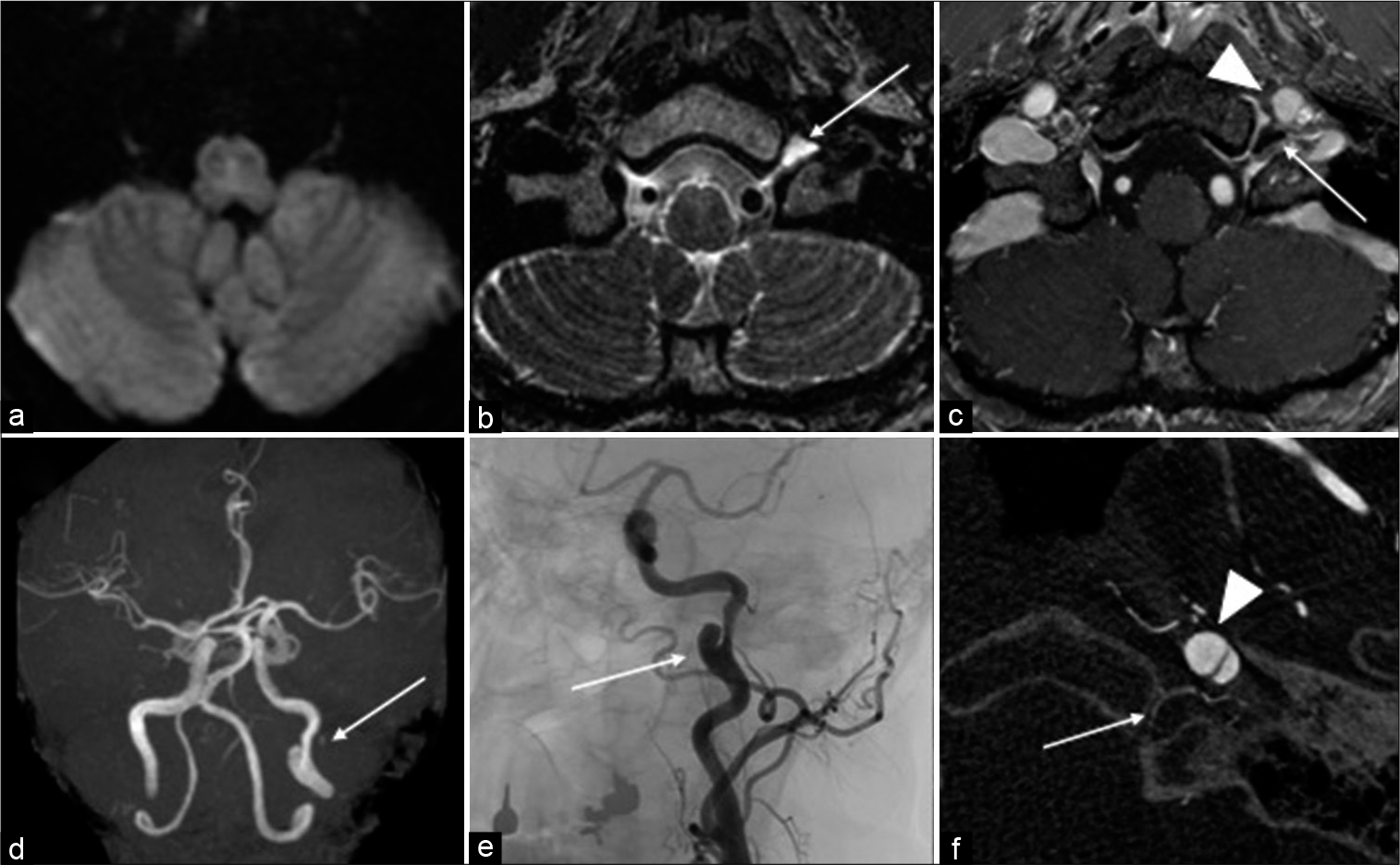
Figure 1:
(a) Diffusion-weighted MRI showing no lesion in the medulla oblongata including the hypoglossal nerve nucleus. (b) T2-CUBE MRI showing false lumen of ICAD compressing the hypoglossal nerve tube, and the perineural space in the left hypoglossal canal is dilated (arrow). (c) T1-weighted MRI with contrast-enhancement showing dissected false cavity (arrowhead) compressing the distal side of the hypoglossal canal (arrow). (d) MRA showing a left ICAD with a false lumen (arrow). (e) Angiography showing a left ICAD (arrow). (f) Cone-beam CT showing that the false lumen (arrowhead) is protruding to the direction of the hypoglossal canal (arrow). Direct compression is thought to be causing hypoglossal nerve palsy.
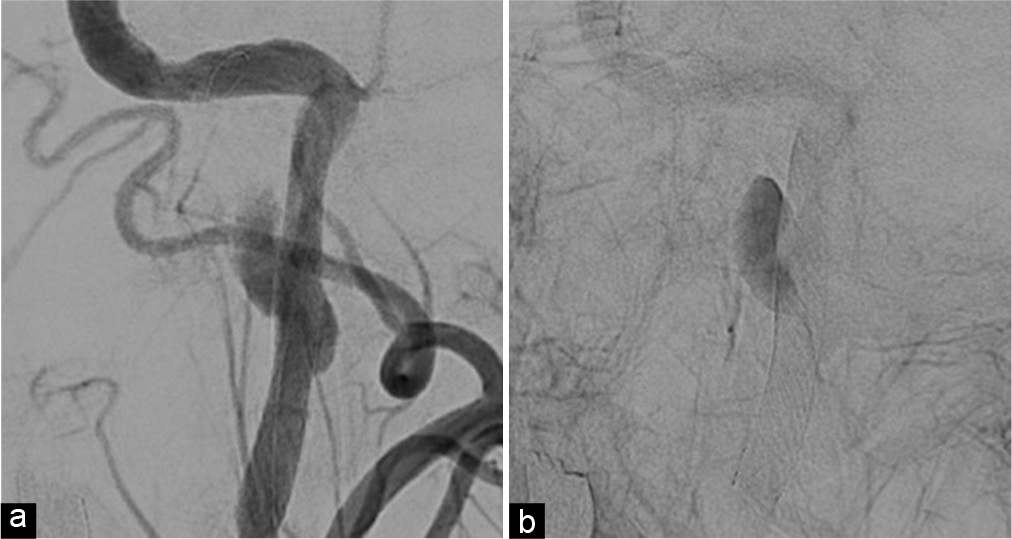
Although medical treatment was continued, his headache worsened, and the neurological symptoms were not improved. Therefore, CAS was performed on the 19th day to thrombose the false lumen and decompress the hypoglossal nerve. A 6Fr guiding catheter (Axcelguide MSK, Medikit, Tokyo, Japan) was inserted through the right brachial artery and a 4-6Fr catheter (Dymon catheter, Silux, Saitama, Japan) was placed in the left common carotid artery. An embolic protection device (FilterWire EZ, Boston Scientific, MA) was advanced through the lesion and a filter was deployed in the ICA at the petrous portion. A carotid stent (Wallstent, Boston Scientific) was advanced to the dissected portion and deployed. Angiography immediately after stenting showed dilatation of the true lumen and congestion of contrast medium in the false lumen [
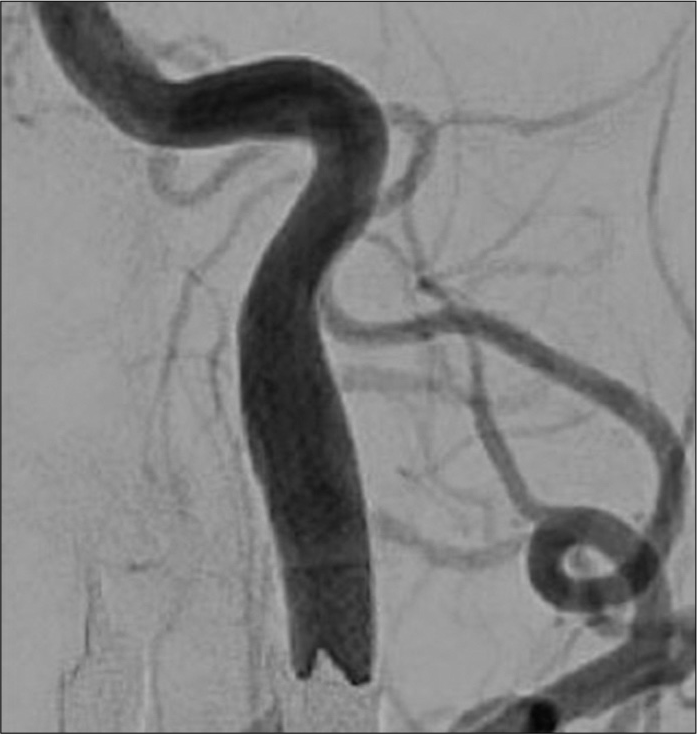
His headache gradually improved after CAS and disappeared on the 25th day. His postoperative course was uneventful and he was discharged on the 26th day. No improvement of hypoglossal nerve palsy was observed at the time of discharge. At 1 month after discharge, neurological symptoms, such as tongue deviation and dysarthria, improved. Cerebral angiography performed at month 6 showed well-dilated ICA and disappearance of false lumen [
DISCUSSION
Spontaneous ICAD is a relatively rare disease and incidence is reported to be 1.72–2.9/100,000 population.[
MRI, MRA, CT angiography (CTA), and cerebral angiography have been used for diagnosis of ICAD. However, in cases of ICAD presenting with the lower cranial nerve palsy, nerve pathways cannot be visualized, and the diagnosis is often based on clinical symptoms. In the present case, angiography showed ICAD with a false lumen, and CBCT was used to visualize the hypoglossal canal and ICAD. CBCT showed ICAD at the level of the hypoglossal canal [
The most common treatment for ICAD is anticoagulant or antiplatelet treatment and there is no significant difference in the outcome between these two treatments.[
CONCLUSION
CAS may be an effective treatment for the lower cranial nerve palsy caused by compression by a false lumen of ICAD. Decompression of the nerve by CAS leads to early improvement of symptoms.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arnoldner C, Riss D, Wagenblast J, Starlinger V, Hamzavi JS. Tenth and twelfth nerve palsies in a patient with internal carotid artery dissection mistaken for cervical mass lesion. Skull Base. 2010. 20: 301-4
2. Bradac GB, Kaernbach A, Bolk-Weischedel D, Finck GA. Spontaneous dissecting aneurysm of cervical cerebral arteries. Report of six cases and review of the literature. Neuroradiology. 1981. 21: 149-54
3. Cruciata G, Parikh R, Pradhan M, Shah J, Greif E, Stein EG. Internal carotid artery dissection and pseudoaneurysm formation with resultant ipsilateral hypoglossal nerve palsy. Radiol Case Rep. 2017. 12: 371-5
4. Dal Pozzo G, Mascalchi M, Fonda C, Cadelo M, Ronchi O, Inzitari D. Lower cranial nerve palsy due to dissection of the internal carotid artery: CT and MR imaging. J Comput Assist Tomogr. 1989. 13: 989-95
5. Davies L. A case of vagal palsy due to dissecting aneurysm of the carotid artery. Med J Aust. 1987. 147: 352-3
6. Giroud M, Fayolle H, Andre N, Dumas R, Becker F, Martin D. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994. 57: 1443
7. Goldberg HI, Grossman RI, Gomori JM, Asbury AK, Bilaniuk LT, Zimmerman RA. Cervical internal carotid artery dissecting hemorrhage: Diagnosis using MR. Radiology. 1986. 158: 157-61
8. Goodman JM, Zink WL, Cooper DF. Hemilingual paralysis caused by spontaneous carotid artery dissection. Arch Neurol. 1983. 40: 653-4
9. Guy N, Deffond D, Gabrillargues J, Carriere N, Dordain G, Clavelou P. Spontaneous internal carotid artery dissection with lower cranial nerve palsy. Can J Neurol Sci. 2001. 28: 265-9
10. Hanyu S, Sato H, Tanaka Y. Isolated hypoglossal nerve palsy due to spontaneous dissection of the internal carotid artery. Neurol Med. 1995. 42: 470-2
11. Havelius U, Hindfelt B, Brismar J, Cronqvist S. Carotid fibromuscular dysplasia and paresis of lower cranial nerves (Collect-Sicard syndrome). Case report. J Neurosurg. 1982. 56: 850-3
12. Heckmann JG, Tomandl B, Duhm C, Stefan H, Neundörfer B. Collet-Sicard syndrome due to coiling and dissection of the internal carotid artery. Cerebrovasc Dis. 2000. 10: 487-8
13. Hommel M, Pollak P, Gaio JM, Pellat J, Perret J, Chateau R. Paralysis of the hypoglossal nerve caused by 2 aneurysms and a dissecting aneurysm of the internal carotid artery. Rev Neurol (Paris). 1984. 140: 415-21
14. Isildak H, Karaman E, Ozdogan A, Ibrahimov M, Yilmaz M. Unusual manifestations of bilateral carotid artery dissection: Dysphagia and hoarseness. Dysphagia. 2010. 25: 338-40
15. Klossek JM, Vandenmarq P, Neau JP, Fontanel JP. Unilateral lower cranial nerve palsies due to spontaneous internal carotid artery dissection. Ann Otol Rhinol Laryngol. 1994. 103: 413-5
16. Lee VH, Brown RD, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection a population-based study. Neurology. 2006. 67: 1809-12
17. Lieschke GJ, Davis S, Tress BM, Ebeling P. Spontaneous internal carotid artery dissection presenting as hypoglossal nerve palsy. Stroke. 1988. 19: 1151-5
18. Majeed A, Ribeiro NP, Ali A, Hijazi M, Farook H. A rare presentation of spontaneous internal carotid artery dissection with Horner’s syndrome, VIIth Xth and XIIth nerve palsies. Oxf Med Case Rep. 2016. 2016: 255-8
19. Markus HS, Levi C, King A, Madigan J, Norris J. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: The cervical artery dissection in stroke study (CADISS) randomized clinical trial final results. JAMA Neurol. 2019. 76: 657-64
20. Mes M, Palczewski P, Szczudlik P, Łusakowska A, Maj E, Gawel M. Hypoglossal nerve palsy as an isolated syndrome of internal carotid artery dissection: A review of the literature and a case report. Neurol Neurochirurg Polska. 2018. 52: 731-5
21. Mizutani S, Tsukuura R, Matsumura K, Watanabe M, Hanakawa I, Kamata T. Villaret’s syndrome caused by internal carotid artery dissection. Rinsho Shinkeigaku. 2011. 51: 608-11
22. Mokri B, Piepgras DG, Wiebers DO, Houser OW. Familial occurrence of spontaneous dissection of the internal carotid artery. Stroke. 1987. 18: 246-51
23. Mokri B, Schievink WI, Olsen KD, Piepgras DG. Spontaneous dissection of the cervical internal carotid artery. Presentation with lower cranial nerve palsies. Arch Otolaryngol Head Neck Surg. 1992. 118: 431-5
24. Moussouttas M, Tuhrim S. Spontaneous internal carotid artery dissection with isolated vagus nerve deficit. Neurology. 1998. 51: 317-8
25. Murakami Y, Oda K, Konno Y, Matsumoto Y, Saito K. Successfully treated with endovascular therapy against lower cranial nerve paresis caused by spontaneous dissection of the cervical internal carotid artery: A case report. J Neuroendovasc Ther. 2016. 10: 30-5
26. Nguyen TT, Zhang H, Dziegielewski PT, Seemann R. Vocal cord paralysis secondary to spontaneous internal carotid dissection: Case report and systematic review of the literature. J Otolaryngol Head Neck Surg. 2013. 42: 34
27. Nusbaum AO, Som PM, Dubois P, Silvers AR. Isolated vagal nerve palsy associated with a dissection of the extracranial internal carotid artery. AJNR Am J Neuroradiol. 1998. 19: 1845-7
28. Panisset M, Eidelman BH. Multiple cranial neuropathy as a feature of internal carotid artery dissection. Stroke. 1990. 21: 141-7
29. Sturzenegger M, Huber P. Cranial nerve palsies in spontaneous carotid artery dissection. J Neurol Neurosurg Psychiatry. 1993. 56: 1191-9
30. Vaes M, Sellitti E, Sukkarieh F, Vanhaeverbeek M. A carotid artery dissection presenting with dysphagia due to a dilation of upper oesophagus. Acta Neurol Belg. 2007. 107: 91-3
31. Waespe W, Niesper J, Imhof HG, Valavanis A. Lower cranial nerve palsies due to internal carotid dissection. Stroke. 1988. 19: 1561-4
32. Xianjun H, Zhiming Z. A systematic review of endovascular management of internal carotid artery dissections. Int Neurol. 2013. 1: 164-70
33. Zeleňák K, Zeleňáková J, Deriggo J, Kurča E, Kantorová E, Poláček H. Treatment of cervical internal carotid artery spontaneous dissection with pseudoaneurysm and unilateral lower cranial nerves palsy by two silk flow diverters. Cardiovasc Intervent Radiol. 2013. 36: 1147-50