- Department of Neurosurgery, NTT Medical Center Tokyo, Tokyo, Japan
- Department of Cerebrovascular Medicine, NTT Medical Center Tokyo, Tokyo, Japan.
Correspondence Address:
Masafumi Segawa, Department of Neurosurgery, NTT Medical Center Tokyo, Tokyo, Japan.
DOI:10.25259/SNI_1021_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masafumi Segawa1, Tomohiro Inoue1, Sho Tsunoda1, Takuya Kanamaru2, Seiji Okubo2. Carotid endarterectomy for acute carotid thrombosis after carotid artery stenting with CASPER Rx® stent: A case report. 27-Jan-2023;14:25
How to cite this URL: Masafumi Segawa1, Tomohiro Inoue1, Sho Tsunoda1, Takuya Kanamaru2, Seiji Okubo2. Carotid endarterectomy for acute carotid thrombosis after carotid artery stenting with CASPER Rx® stent: A case report. 27-Jan-2023;14:25. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12132
Abstract
Background: Acute carotid stent thrombosis (ACST) is a rare but devastating complication for carotid artery stenting (CAS). That requires early diagnosis and immediate treatment. Although administration of drugs or endovascular treatment is the most widely used approach for patients with ACST, there is no consensus on the standard treatment of this disease.
Case Description: The present study reports on an 80-year-old female patient with the right internal carotid artery stenosis (ICS) that had been followed up by ultrasonography for 8 years. Although the optimal medical treatment was followed, the patient’s right ICS worsened, and the patient was subsequently hospitalized for CAS. On the 12th day after CAS, left paralysis and dysarthria were observed. Head magnetic resonance imaging (MRI) showed acute obstruction of the stent and scattered cerebral infarction in the right cerebral hemisphere caused possibly by the discontinuation of temporary antiplatelet drug therapy as a means to prepare for embolectomy of the femoral artery. Stent removal and carotid endarterectomy (CEA) were selected as the appropriate treatment approach. CEA was performed with the precaution of stent removal and distal embolism, and complete recanalization was obtained. Postoperative head MRI showed no new findings of cerebral infarction, and the patients remained symptom-free after 6 months of postoperative follow-up.
Conclusion: Stent removal with CEA could be an appropriate curative option in some cases with ACST except in patients at high risk of CEA and in the chronic phase after CAS.
Keywords: Carotid artery stenting, Carotid endarterectomy, External carotid artery, Internal carotid artery stenosis, Stent thrombosis
INTRODUCTION
Carotid artery stenting (CAS) is a well-known treatment for carotid artery stenosis. The number of CAS cases has been increasing in Japan during the past years. Acute carotid stent thrombosis (ACST) is a rare condition with devastating complications requiring urgent treatment,[
In this case report, we describe a successful revascularization case by carotid endarterectomy (CEA) in a patient with ACST.
CASE DESCRIPTION
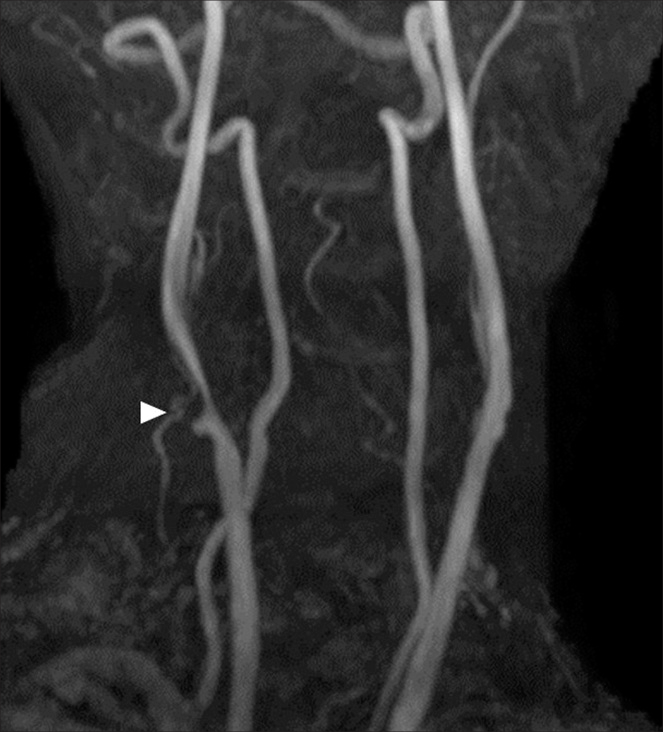
An 80-year-old female patient with hypertension and dyslipidemia had been followed-up by carotid ultrasonography of the right internal carotid artery stenosis (ICS) for 8 years. Although an optimal drug treatment was employed, consisting of aspirin (100 mg/day) and clopidogrel (75 mg/day), ICS gradually worsened, and the patient was eventually hospitalized for CAS. Magnetic resonance imaging (MRI) showed severe stenosis at the right carotid bifurcation [
Figure 2:
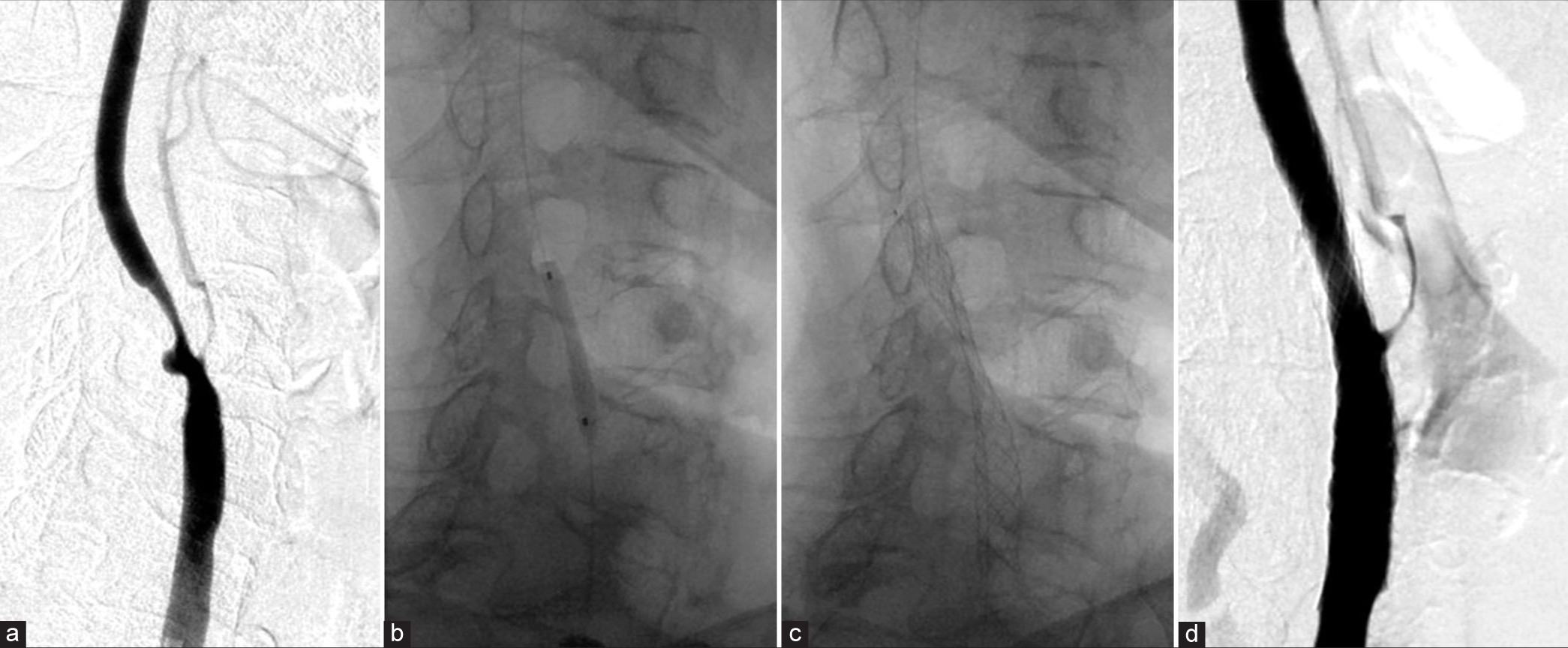
(a) Lateral view of the right common carotid artery on a preoperative angiogram focused on the neck. (b) The stenosis of the right internal carotid artery was predilated with a 3 × 20 mm dilation balloon. (c) A 7 × 25 mm self-expandable carotid stent (CASPER Rx®) was placed. (d) After post-dilation, the angiogram revealed good revascularization.
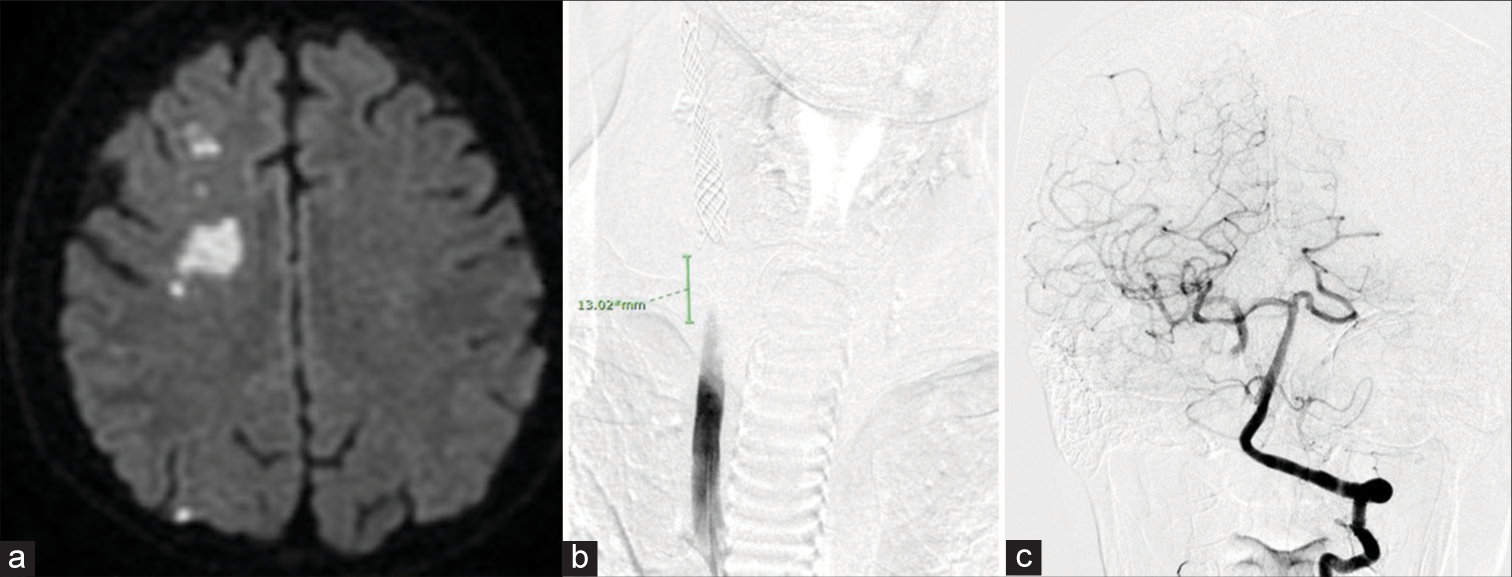
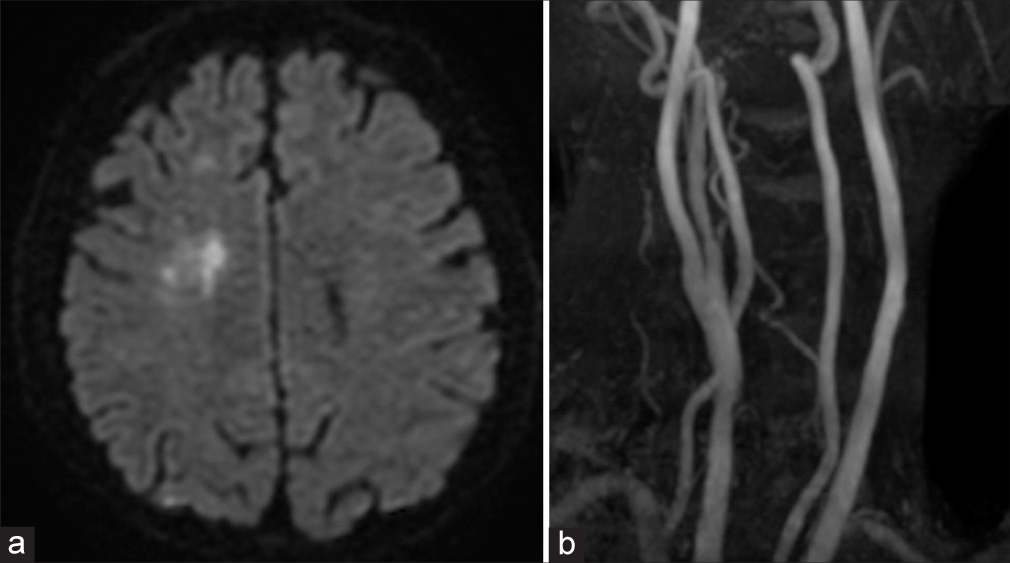
Administration of clopidogrel was resumed on POD10. On POD12, left hemiplegia and dysarthria were suddenly observed (National Institute Health of Stroke Scale, scores 6), and MRI showed scattered infarctions of the right cerebral hemisphere and occlusion of the CCA [
Figure 4:
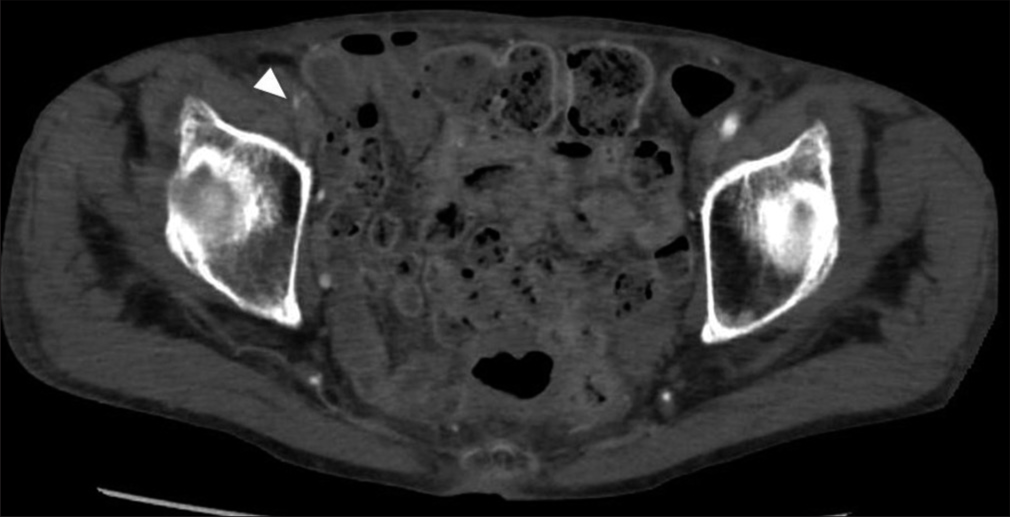
(a) MRI showed scattered infarction of the right cerebral hemisphere and (b) the occlusion of the right common carotid artery was (CCA) observed on angiography. (c) A patency of the distal part of the right internal carotid artery (cavernous portion) was confirmed on a preoperative angiogram.
Figure 5:
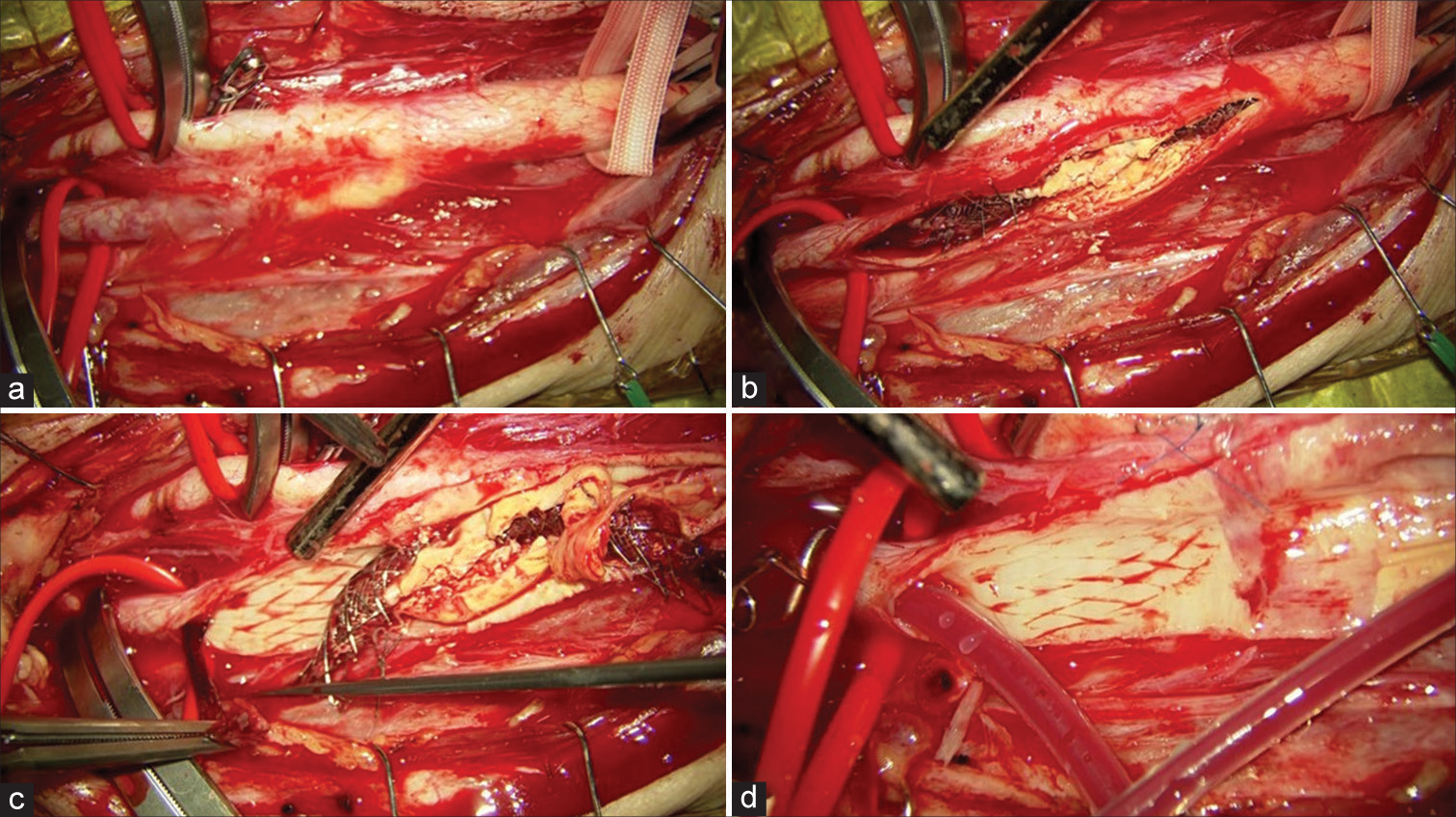
Operative view. (a) The right common carotid artery, internal carotid artery, external carotid artery, and superior thyroid artery were clamped temporally before arteriotomy. (b) The carotid stent was cut with arteriotomy. (c) A thrombus in the stent was observed. (d) Compression marks due to the stent were observed on the intima of the blood vessel.
Figure 6:
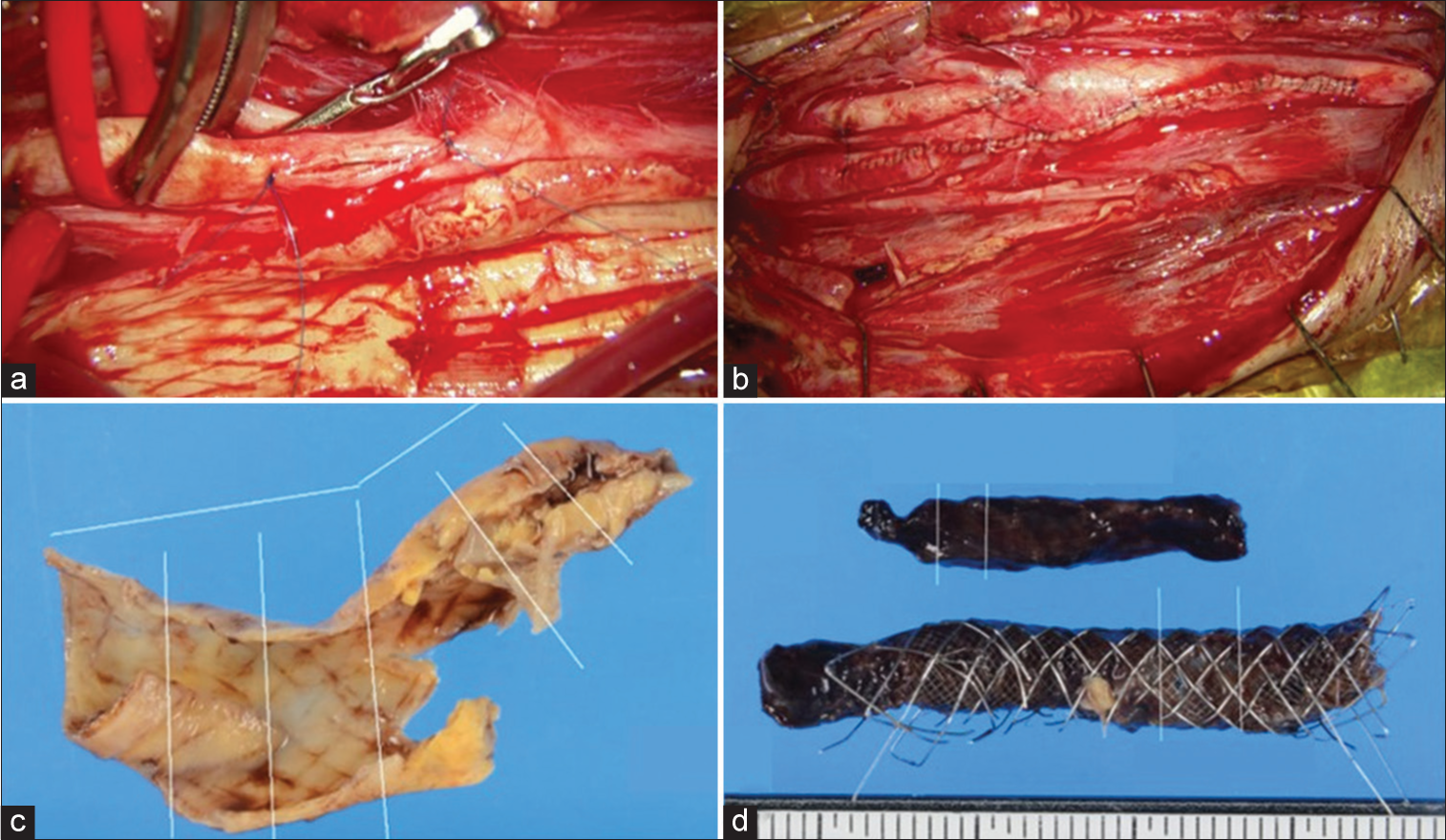
Operative view. (a) An arteriotomy was also performed in the external carotid artery. (b) The artery was sutured continuously with 6-0 proline on both sides. (c and d) Histopathologically, a part of the intima and media was collected, and there was no evidence of plaque protrusion into the stent.
Supplemental Video
DISCUSSION
CEA was performed to treat in-stent thrombosis after CAS, and our findings revealed that a good clinical outcome was achieved. At present, CAS is used as an alternative treatment to CEA for carotid artery stenosis. The efficacy and safety of CAS have increased according to the results of randomized controlled trials.[
Possible coping strategies for ACST include the following: (1) administration of additional drugs such as argatroban; (2) thrombolytic therapy with rt-PA and urokinase; (3) endovascular treatment such as thrombus recovery; (4) reangioplasty such as percutaneous transluminal angioplasty (PTA) and re-CAS; and (5) stent removal and carotid thromboendarterectomy. Complications of re-angioplasty include the possibility of distal embolism and re-thrombosis associated with deterioration of the stent structure. Although the long-term prognosis after revascularization for ACST is not clear, Masuo et al. reported a case of 60% re-stenosis 9 months after the PTA.[
We must evaluate the patency of distal ICA for ICA occlusion in cases of CEA. In the present case, the ipsilateral retrograde cavernous to petrous ICA opacification was noted through cross-flow in delayed arterial phase of contralateral carotid injection. In the series of CEA for ICA occlusion (with patent CCA and ECA), the retrograde reflux visualization of occluded ICA back to the cavernous portion or further back to the petrous portion in preoperative angiography has been associated with 50% and 71% successful reopening of ICA, respectively.[
Meanwhile, endovascular treatments might be advantageous compared to CEA from the standpoint of time, because treatments can be performed as soon as the diagnosis is made. If angiography detects an intracranial artery occlusion, endovascular surgery should be the first choice for ACST. However, re-angioplasty or CEA should also be considered depending on the available manpower, endovascular staff, etc.
The CASPER Rx® (TCD-15152) stent (Terumo Co, Tokyo, Japan) is a self-expandable nitinol stent with a dual-layer structure of tubular mesh.[
CONCLUSION
Stent removal with CEA could be an appropriate curative option in some suitable cases with ACST. Indication should be carefully considered in patients at high risk of CEA and in the chronic phase after CAS. Long-term surveillance is necessary to assess the durability of this procedure.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available online at
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY VIDEO
References
1. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016. 374: 1021-31
2. Broussalis E, Griessenauer C, Mutzenbach S, Pikija S, Jansen H, Stevanovic V. Reduction of cerebral DWI lesion burden after carotid artery stenting using the casper stent system. J Neurointerv Surg. 2019. 11: 62-7
3. Chaturvedi S, Sohrab S, Tselis A. Carotid stent thrombosis: Report of 2 fatal cases. Stroke. 2001. 32: 2700-2
4. Cheneau E, Leborgne L, Mintz GS, Kotani J, Pichard AD, Satler LF. Predictors of subacute stent thrombosis: Results of a systematic intravascular ultrasound study. Circulation. 2003. 108: 43-7
5. Hu W, Wang L, Wang G. Acute in-stent thrombosis after carotid angioplasty and stenting: A case report and literature review. Interv Neurol. 2018. 7: 265-70
6. Markatis F, Petrosyan A, Abdulamit T, Bergeron P. Acute carotid stent thrombosis: A case of surgical revascularization and review of treatment options. Vascular. 2012. 20: 217-20
7. Masuo O, Terada T, Matsuda Y, Ogura M, Tsumoto T, Yamaga H. Successful recanalization by in-stent percutaneous transluminal angioplasty with distal protection for acute carotid stent thrombosis. Neurol Med Chir (Tokyo). 2006. 46: 495-9
8. McCormick PW, Spetzler RF, Bailes JE, Zabramski JM, Frey JL. Thromboendarterectomy of the symptomatic occluded internal carotid artery. J Neurosurg. 1992. 76: 752-8
9. Moulakakis KG, Lazaris AM. Emergent carotid stent removal after carotid stent thrombosis. Ann Vasc Surg. 2018. 46: 401-6
10. Moulakakis KG, Mylonas SN, Lazaris A, Tsivgoulis G, Kakisis J, Sfyroeras GS. Acute carotid stent thrombosis: A comprehensive review. Vasc Endovasc Surg. 2016. 50: 511-21
11. Setacci C, de Donato G, Setacci F, Chisci E, Cappelli A, Pieraccini M. Surgical management of acute carotid thrombosis after carotid stenting: A report of three cases. J Vasc Surg. 2005. 42: 993-6
12. Wissgott C, Schmidt W, Brandt C, Behrens P, Andresen R. Preliminary clinical results and mechanical behavior of a new double-layer carotid stent. J Endovasc Ther. 2015. 22: 634-9