- Department of Neurosurgery, Nagareyama Central Hospital, Nagareyama City, Japan.
Correspondence Address:
Yuichi Takahashi, Department of Neurosurgery, Nagareyama Central Hospital, Nagareyama City, Japan.
DOI:10.25259/SNI_1222_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuichi Takahashi, Tetsuhiro Higashida, Takanori Uchida, Saiko Watanabe, Ryuzaburo Kanazawa. Carotid endarterectomy with stent removal for recurring in-stent restenosis: A case report and literature review. 06-May-2022;13:192
How to cite this URL: Yuichi Takahashi, Tetsuhiro Higashida, Takanori Uchida, Saiko Watanabe, Ryuzaburo Kanazawa. Carotid endarterectomy with stent removal for recurring in-stent restenosis: A case report and literature review. 06-May-2022;13:192. Available from: https://surgicalneurologyint.com/surgicalint-articles/11580/
Abstract
Background: Percutaneous transcatheter angioplasty (PTA) and carotid artery stenting (CAS) are often performed repeatedly for in-stent restenosis (ISR) after CAS. Only a few reports describe the treatment for repeated ISR. Furthermore, only a few reports describe carotid endarterectomy (CEA) after CAS; thus, the evidence for this procedure is insufficient.
Case Description: Herein, we describe a case in which CEA with stent removal was performed in a patient with repeated ISR after CAS. A 78-year-old man presented with dysarthria and slight left limb weakness. CAS was performed for the right internal carotid artery stenosis. ISR occurred again and PTA and stenting were performed. After the second CAS, ISR occurred again. CEA with stent removal was performed. After the CEA with stent removal, the patient experienced no restenosis or other complications.
Conclusion: CEA with stent removal can be a good option for treating repeated ISR after CAS.
Keywords: Carotid artery stent, Carotid endarterectomy, In-stent restenosis, Internal carotid artery stenosis, Neointimal hyperplasia
INTRODUCTION
The rates of in-stent restenosis (ISR) after carotid artery stenting (CAS) are between 3% and 10% and ISR is associated with ipsilateral cerebral infarction.[
CASE REPORT
A 78-year-old man with hypertension, Type 2 diabetes mellitus, and dyslipidemia presented with dysarthria and slight left limb weakness. Diffusion-weighted imaging revealed multiple small cerebral infarctions spreading cortically and a watershed area in the right brain hemisphere [
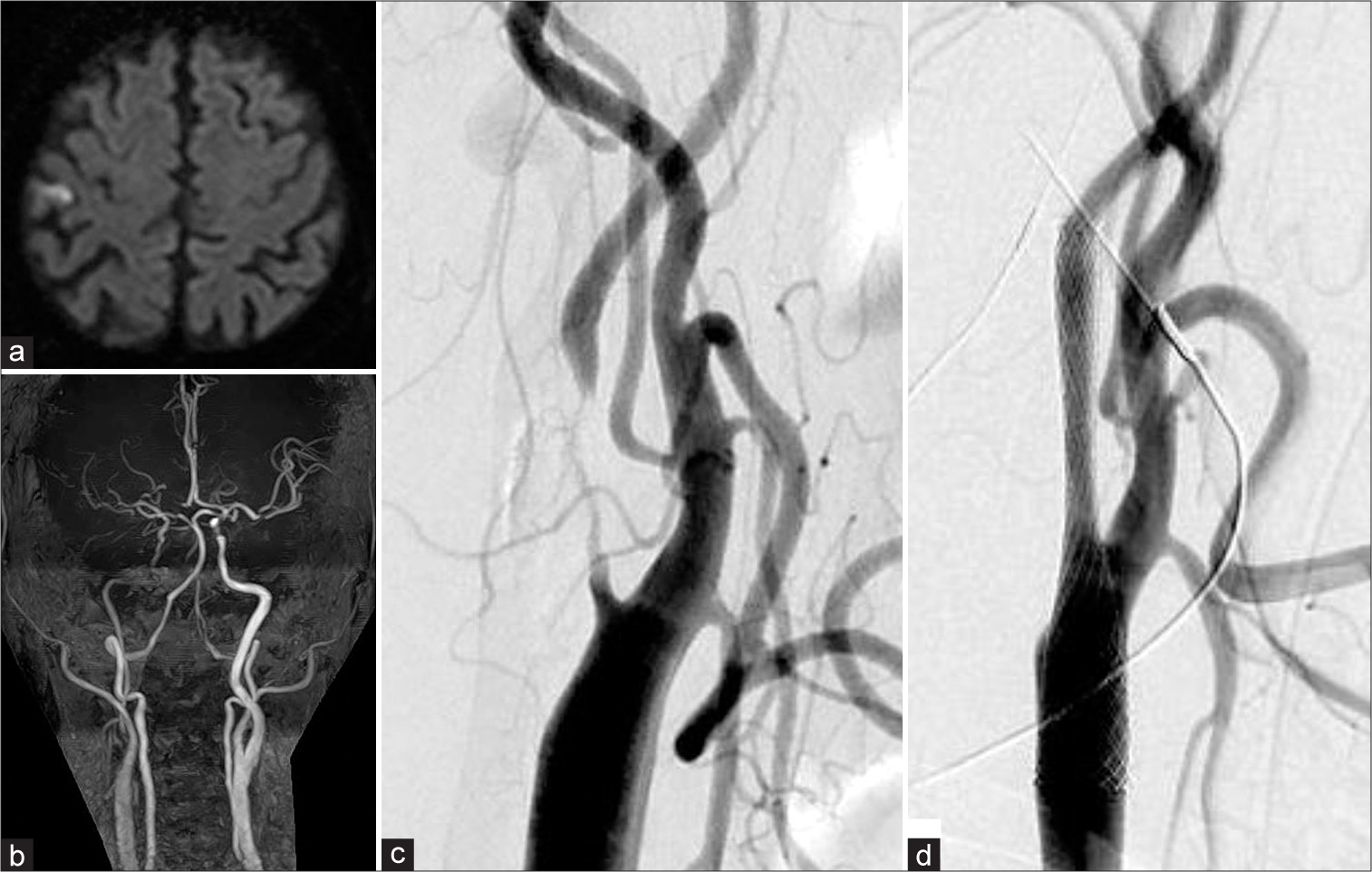
Figure 1:
(a) Diffusion-weighted imaging at initial presentation showing a right frontal infarction. (b) Magnetic resonance angiography showing severe stenosis on the right side. (c) Preoperative angiography showing severe right internal carotid stenosis. (d) Angiography after carotid artery stenting.
CAS was performed 1 month after symptom onset. Although a staged CAS was planned, plaque protrusion occurred just after PTA; therefore, we deployed a closed-cell stent (Carotid WallStent 10 × 24 mm, Boston Scientific, Marlborough, MA, USA) [
Figure 2:
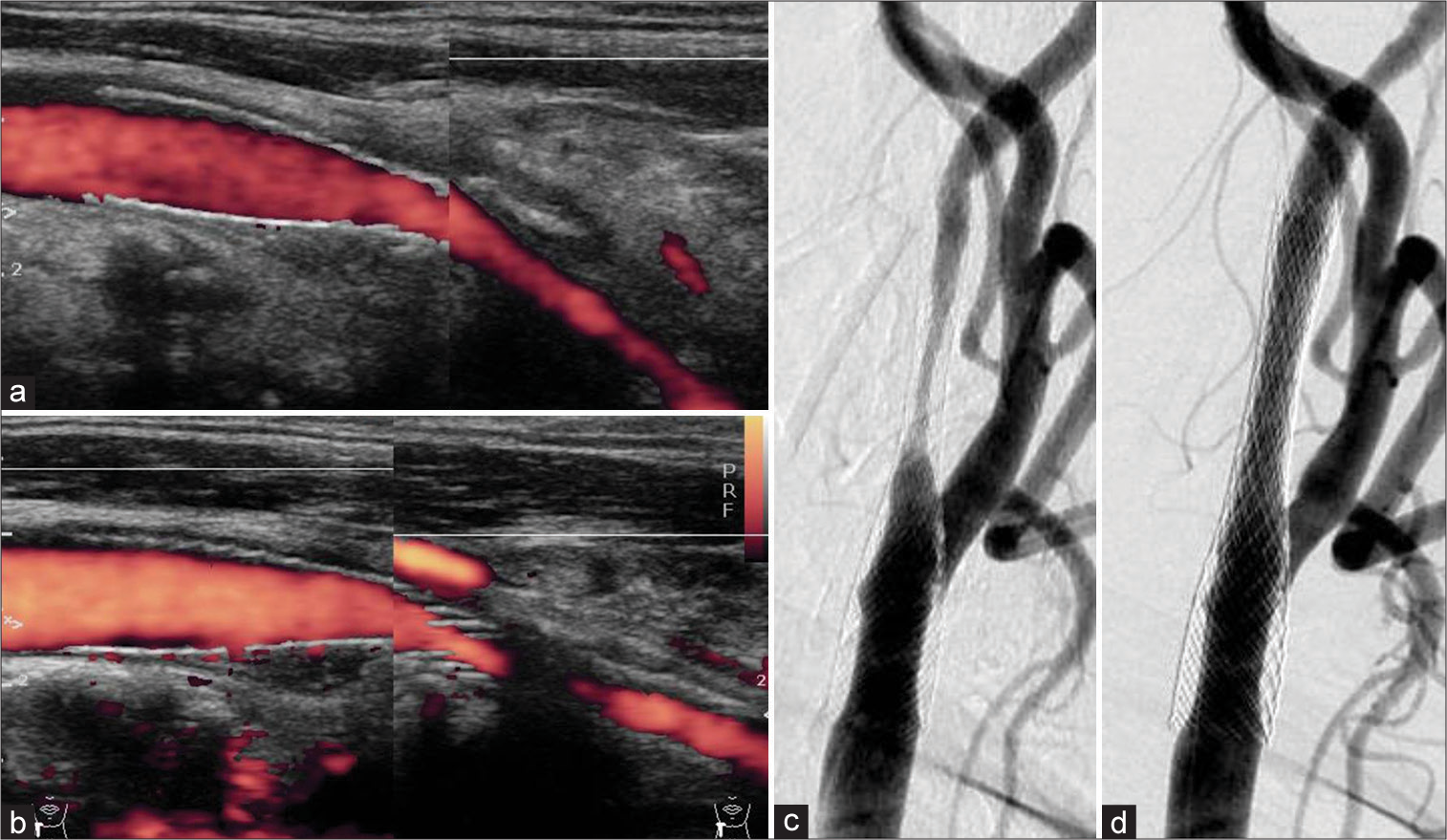
(a) After carotid artery stenting (CAS), the postoperative carotid echography revealed no stenosis. The peak systolic velocity (PSV) was 62.3 cm/s. (b) A carotid echography 4 months after the first CAS showing in-stent restenosis (ISR). The PSV was over 300 cm/s. (c) Angiography showing ISR 4 months after the first CAS. (d) The angiography after percutaneous transcatheter angioplasty and a second CAS were performed.
Six to 9 months after the second CAS, ISR again worsened; the carotid echography revealed a PSV of 139–317 cm/s [
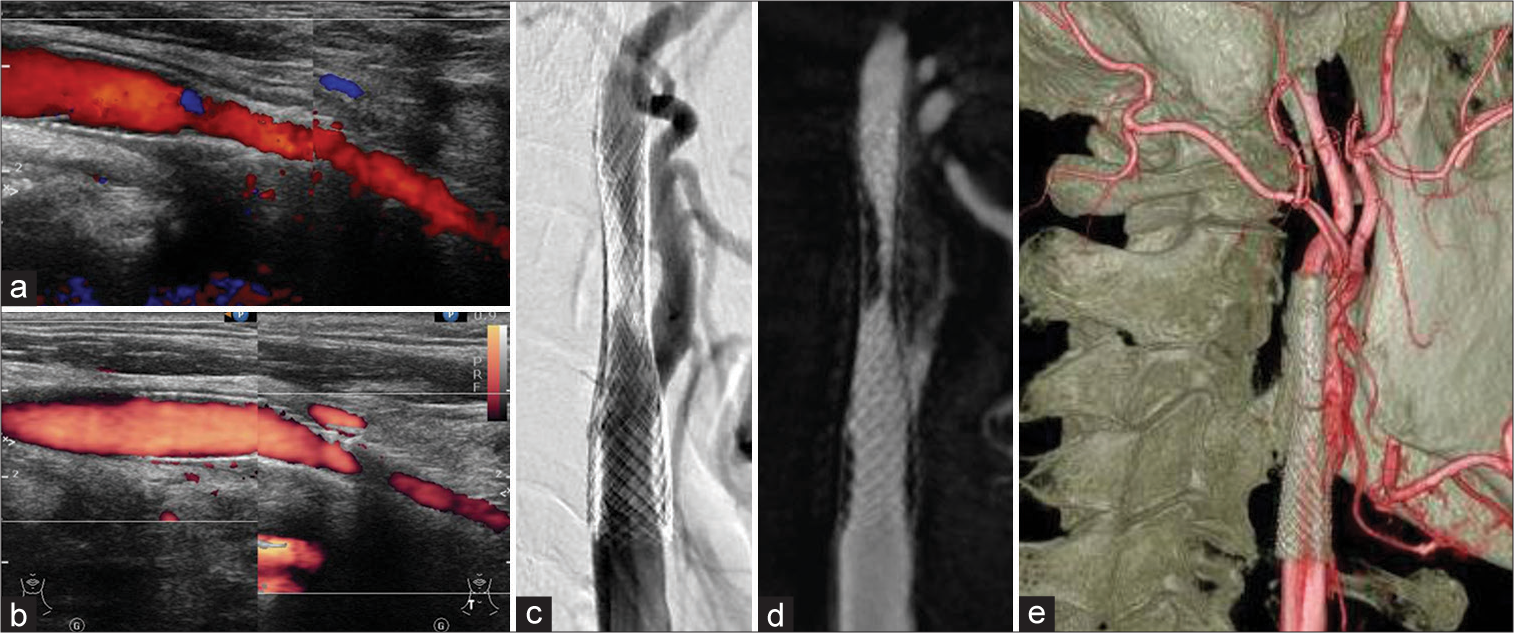
Figure 3:
Worsening in-stent restenosis (ISR) after the second carotid artery stenting is shown. The carotid echography revealed peak systolic velocity changes from 139 cm/s (a) to 317 cm/s (b) 6 to 9 months after the second carotid artery stenting. (c and d) The angiography shows severe ISR. (e) The distal end of the stent location at the C2 level is shown.
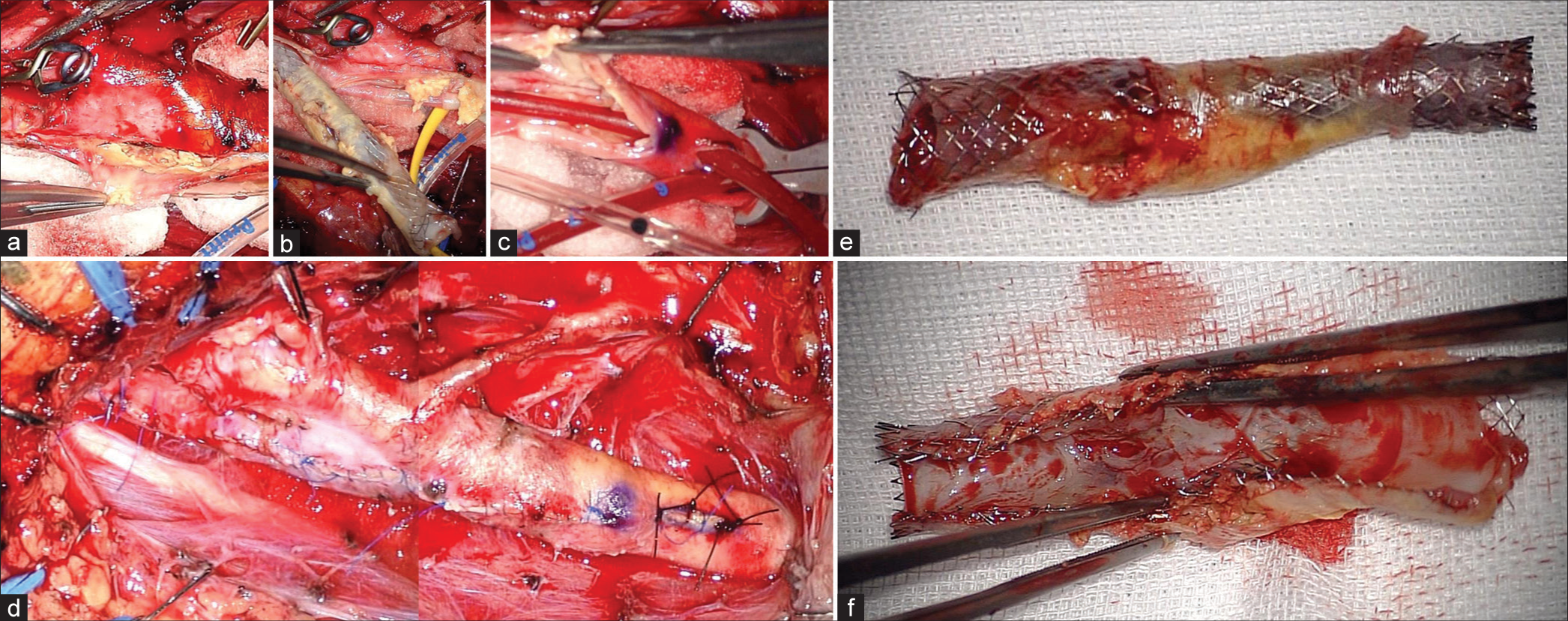
Figure 4:
(a and b) The clear boundary between the plaque, including the stent, and the vessel wall is shown. Normal carotid endarterectomy was performed. (c) Insertion of an internal shunt after removed plaque is shown. (d) The vessel wall could be sutured as normal. (e and f) The stent and plaque are shown. Neointima formed inside the lumen of the stent, causing in-stent restenosis.
DISCUSSION
ISR is a complication that may occur after CAS. Cases of symptomatic or severe restenosis may require retreatment. The rate of cerebral infarction onset on the same side after CAS was 6.6%/3 years in the SAPPHIRE study,[
ISR has three primary causes. First, early postoperative stenosis with plaque protrusion or thrombus on the stent can cause ISR. Second, neointimal hyperplasia caused by non-specific inflammatory responses of blood vessels to the chronic mechanical stimulation of stents can cause ISR.[
CONCLUSION
Conclusions about the optimal intervention for ISR cannot be made yet; however, CEA with stent removal is another good option.[
Our case required repeated treatment for ISR. After a careful discussion about the optimal treatment, we decided to perform CEA with stent removal after the third occurrence of ISR. If a third CAS had been performed, the lumen might have narrowed because of the thickness of the stent itself and ISR might have reoccurred. CEA was problematic for this patient, because of inadequate collateral flow, and insertion of an internal shunt was needed. The distal end of the stent was at the C2 level, and we were unsure if the space would be adequate for insertion of the internal shunt. As a result, CEA was carried out 13 months after the second CAS.
The structure between the intima and media is maintained because the carotid stent is located inside the intima. In earlier studies, a few perioperative technical difficulties for CEA with stent removal were reported.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Enago (www.enago.jp ) for the English language review.
References
1. Arquizan C, Trinquart L, Touboul PJ, Long A, Feasson S, Terriat B. Restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke. 2011. 42: 1015-20
2. Bonati LH, Ederle J, McCabe DJ, Dobson J, Featherstone RL, Gaines PA. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009. 8: 908-17
3. Bonati LH, Gregson J, Dobson J, McCabe DJ, Nederkoorn PJ, van der Worp HB. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): Secondary analysis of a randomised trial. Lancet Neurol. 2018. 17: 587-96
4. Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010. 363: 11-23
5. Clavel P, Hebert S, Saleme S, Mounayer C, Rouchaud A, Marin B. Cumulative incidence of restenosis in the endovascular treatment of extracranial carotid artery stenosis: A meta-analysis. J Neurointerv Surg. 2019. 11: 916-23
6. Dakour-Aridi H, Mathlouthi A, Locham S, Goodney P, Schermerhorn ML, Malas MB. Predictors of midterm high-grade restenosis after carotid revascularization in a multicenter national database. J Vasc Surg. 2020. 71: 1972-81
7. de Borst GJ, Ackerstaff RG, Mauser HW, Moll FL. Operative management of carotid artery in-stent restenosis: first experiences and duplex follow-up. Eur J Vasc Endovasc Surg. 2003. 26: 137-40
8. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol. 2008. 7: 893-902
9. Gonzalez A, Drummond M, McCord S, Garrett HE. Carotid endarterectomy for treatment of in-stent restenosis. J Vasc Surg. 2011. 54: 1167-9
10. Guo Z, Liu C, Huang K, Yu N, Peng M, Starnes BW. Meta-analysis of redo stenting versus endarterectomy for in-stent stenosis after carotid artery stenting. J Vasc Surg. 2021. 73: 1282-9
11. Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008. 358: 1572-9
12. Khan MA, Liu MW, Chio FL, Roubin GS, Iyer SS, Vitek JJ. Predictors of restenosis after successful carotid artery stenting. Am J Cardiol. 2003. 92: 895-7
13. King BN, Scher LA, Lipsitz EC. Refractory in-stent restenosis following carotid artery stenting: A case report and review of operative management. Vasc Endovascular Surg. 2009. 43: 306-11
14. Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB. Restenosis after carotid artery stenting and endarterectomy: A secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. 2012. 11: 755-63
15. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006. 355: 1660-71
16. Megaly M, Alani F, Cheng CI, Ragina N. Risk factors for the development of carotid artery in-stent restenosis: Multivariable analysis. Cardiovasc Revasc Med. 2021. 24: 65-9
17. Miyata H, Nakahara I, Ishii A, Ohta T, Sadamasa N, Matsumoto S. Predictors and stroke risk of restenosis after carotid artery stenting. J Neuroendovascular Ther. 2015. 9: 245-53
18. Müller MD, Gregson J, McCabe DH, Nederkoorn PJ, van der Worp HB, de Borst GJ. Stent design, restenosis and recurrent stroke after carotid artery stenting in the international carotid stenting study. Stroke. 2019. 50: 3013-20
19. Pourier VE, de Borst GJ. Technical options for treatment of in-stent restenosis after carotid artery stenting. J Vasc Surg. 2016. 64: 1486-96
20. Reichmann BL, van Laanen JH, de Vries JP, Hendriks JM, Verhagen HJ, Moll FL. Carotid endarterectomy for treatment of in-stent restenosis after carotid angioplasty and stenting. J Vasc Surg. 2011. 54: 87-92
21. Setacci C, de Donato G, Setacci F, Chisci E, Cappelli A, Pieraccini M. Surgical management of acute carotid thrombosis after carotid stenting: A report of three cases. J Vasc Surg. 2005. 42: 993-6
22. Skelly CL, Gallagher K, Fairman RM, Carpenter JP, Velazquez OC, Parmer SS. Risk factors for restenosis after carotid artery angioplasty and stenting. J Vasc Surg. 2006. 44: 1010-5
23. Texakalidis P, Tzoumas A, Giannopoulos S, Jonnalagadda AK, Jabbour P, Rangel-Castilla L. Risk factors for restenosis after carotid revascularization: A meta-analysis of hazard ratios. World Neurosurg. 2019. 125: 414-24
24. Yamagami H, Sakai N, Matsumaru Y, Sakai C, Kai Y, Sugiu K. Periprocedural cilostazol treatment and restenosis after carotid artery stenting: The retrospective study of in-stent restenosis after carotid artery stenting (ReSISteR-CAS). J Stroke Cerebrovasc Dis. 2012. 21: 193-9
25. Yu LB, Yan W, Zhang Q, Zhao JZ, Zhang Y, Wang R. Carotid endarterectomy for treatment of carotid instent restenosis: Long-term follow-up results and surgery experiences from one single centre. Stroke Vasc Neurol. 2017. 2: 140-6
26. Zheng J, Liu L, Cao Y, Zhang D, Wang R, Zhao J. Carotid endarterectomy with stent removal in management of in-stent restenosis: A safe, feasible, and effective technique. Eur J Vasc Endovasc Surg. 2014. 47: 8-12