- Department of Neurosurgery, Dr. Soeradji Tirtonegoro Central Public Hospital, Klaten, Indonesia
- Department of Neurosurgery, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, Indonesia
- Department of Pathology Anatomy, Dr. Soeradji Tirtonegoro Central Public Hospital, Klaten, Indonesia
Correspondence Address:
Wisnu Baskoro, Department of Neurosurgery, Dr. Soeradji Tirtonegoro Central Public Hospital, Klaten, Indonesia.
DOI:10.25259/SNI_1129_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Wisnu Baskoro1, Muhammad Fakhri Raiyan Pratama1, Ayu Yoniko Christi1, Muhammad Faris2, Eko Agus Subagio2, Pandu Wicaksono2, Bidari Kameswari3. Case of compressive myelopathy due to juvenile xanthogranuloma of cervicothoracic junction in a 28-year-old male. 20-Jan-2023;14:17
How to cite this URL: Wisnu Baskoro1, Muhammad Fakhri Raiyan Pratama1, Ayu Yoniko Christi1, Muhammad Faris2, Eko Agus Subagio2, Pandu Wicaksono2, Bidari Kameswari3. Case of compressive myelopathy due to juvenile xanthogranuloma of cervicothoracic junction in a 28-year-old male. 20-Jan-2023;14:17. Available from: https://surgicalneurologyint.com/surgicalint-articles/12114/
Abstract
Background: Juvenile xanthogranuloma (JXG) is a proliferative disorder of non-Langerhans histiocytes. The lesions typically occur in children as solitary cutaneous lesions, but are only rarely found in adults in their late twenties to thirties. Approximately 5–10% of JXG are extracutaneous in location, with spinal JXG being only rarely encountered. Here, we described a 28-year-old male with an extradural spinal JXG resulting in severe C6– T1 spinal cord compression and a progressive quadriparesis that warranted a decompressive laminectomy/C6–T2 fusion.
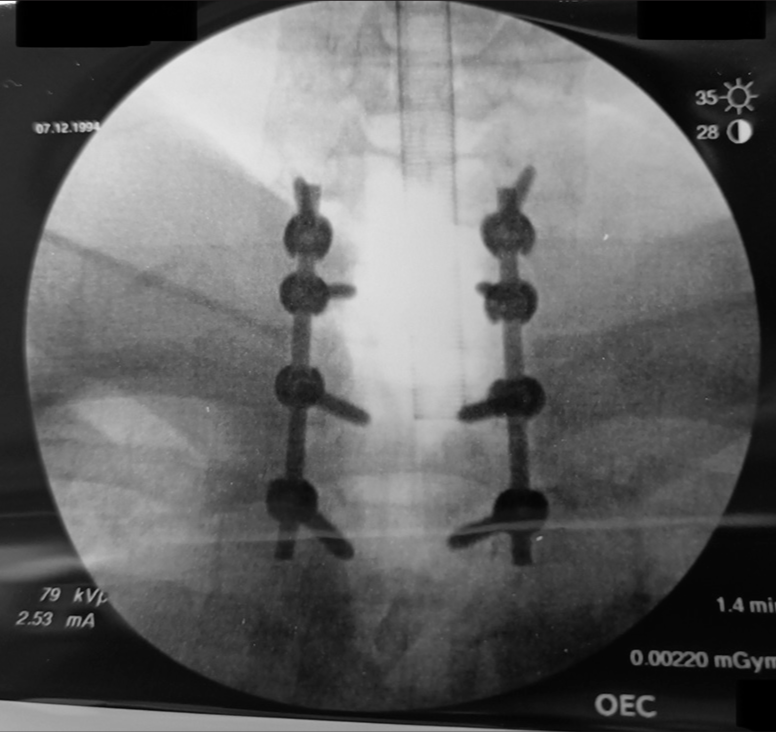
Case Description: A 28-year-old male presented with a progressive quadriparesis of 12 months’ duration that rapidly worsened over the last 3 months. When the MRI revealed severe cord epidural C6–T1 cord compression, the patient successfully underwent a C6–T1 laminectomy for gross total tumor excision followed by a C6–T2 instrumented fusion. The histopathology confirmed the diagnosis of a spinal JXG.
Conclusion: Spinal JXGs in adults are only rarely encountered and should be treated with gross total tumor excision with/without fusion to achieve the best long-term outcomes.
Keywords: Extradural tumor, Gross total removal, Juvenile xanthogranuloma, Laminectomy, Spinal tumor
INTRODUCTION
Juvenile Xanthogranuloma (JXG) is a proliferative disorder of non-Langerhans histiocytes that typically occur in children under the age of one. Often they are solitary cutaneous lesions that spontaneously resorb.[
CASE PRESENTATION
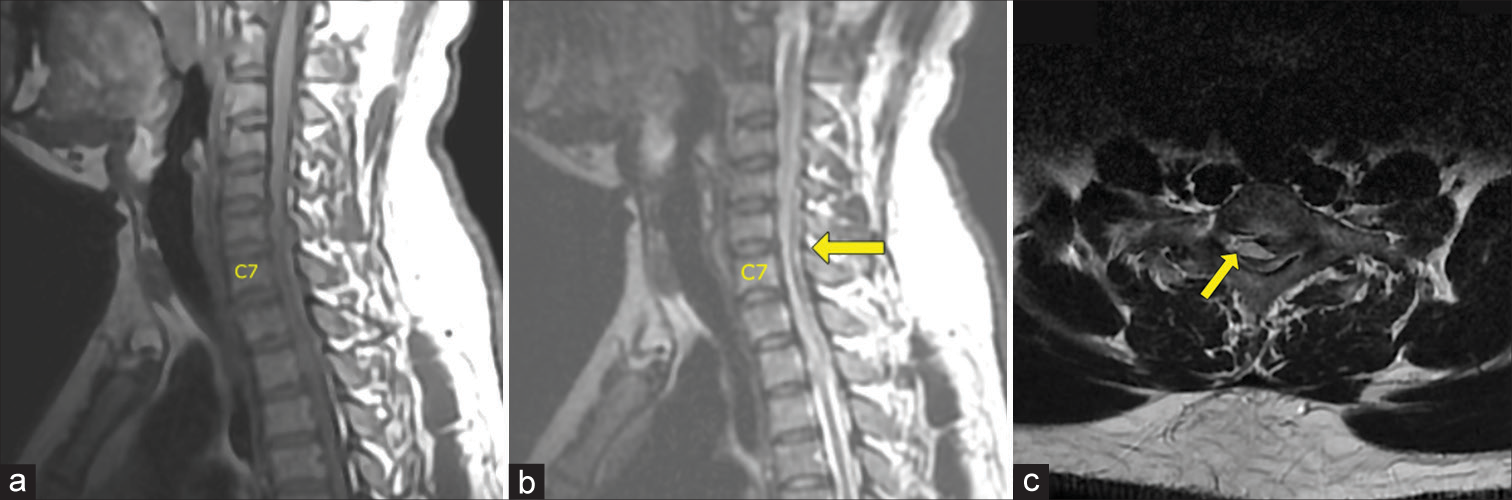
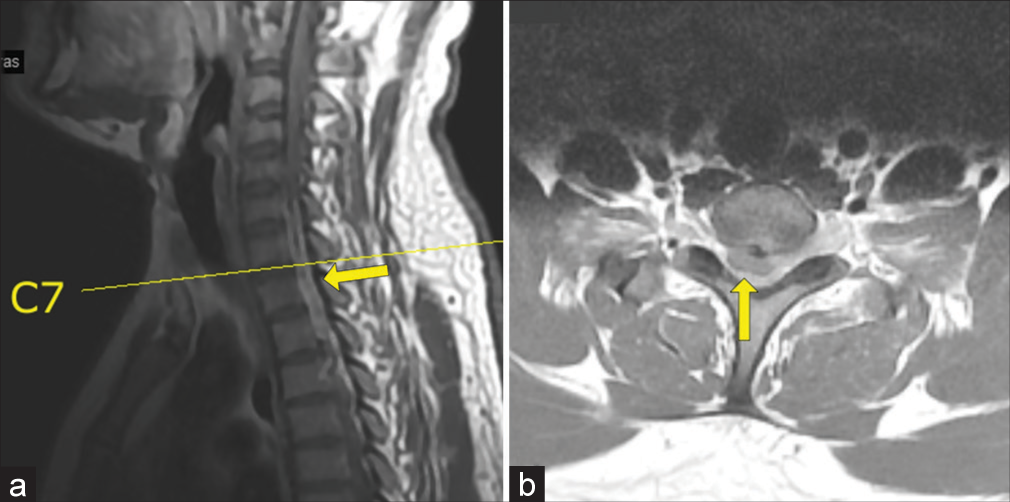
A 28-year-old male presented with neck pain and tingling in both hands for 1 year followed by 3 months of progressive quadriparesis (i.e., 4/5 upper, 1/5 weakness lower extremities, hypoesthesia below C7, and bilateral lower extremity clonus). The MR showed a posterior, extradural, and isointense soft-tissue mass, measuring 1.0 × 0.7 × 3.0 cm extending from C6 to T1 resulting in severe cord compression that did not enhance with contrast [
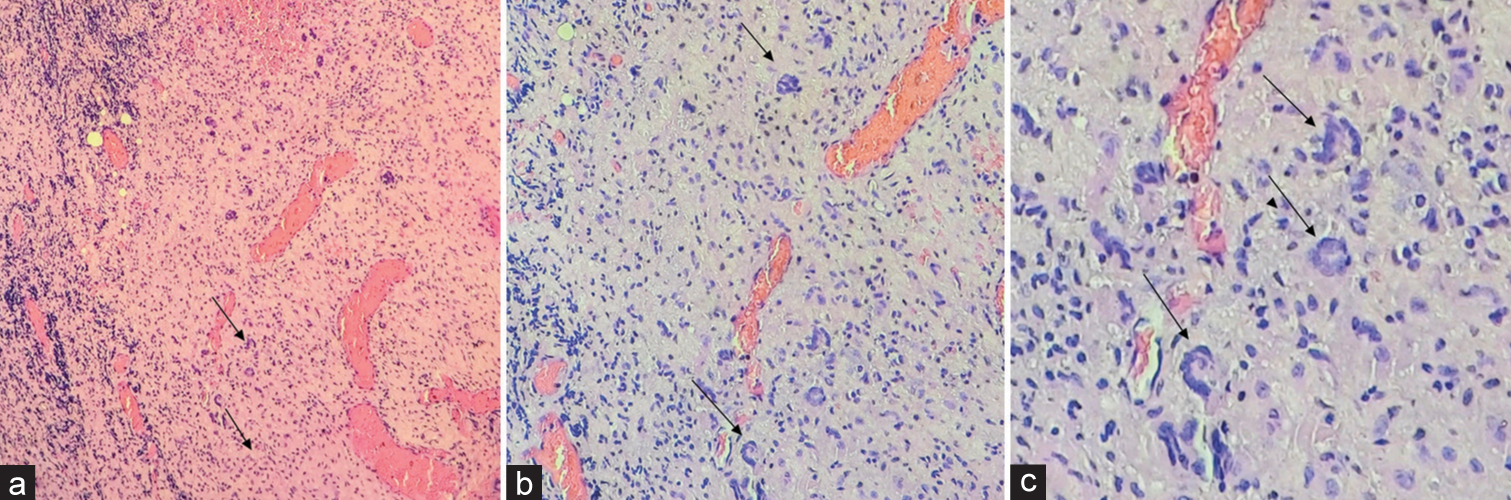
Histopathology examination
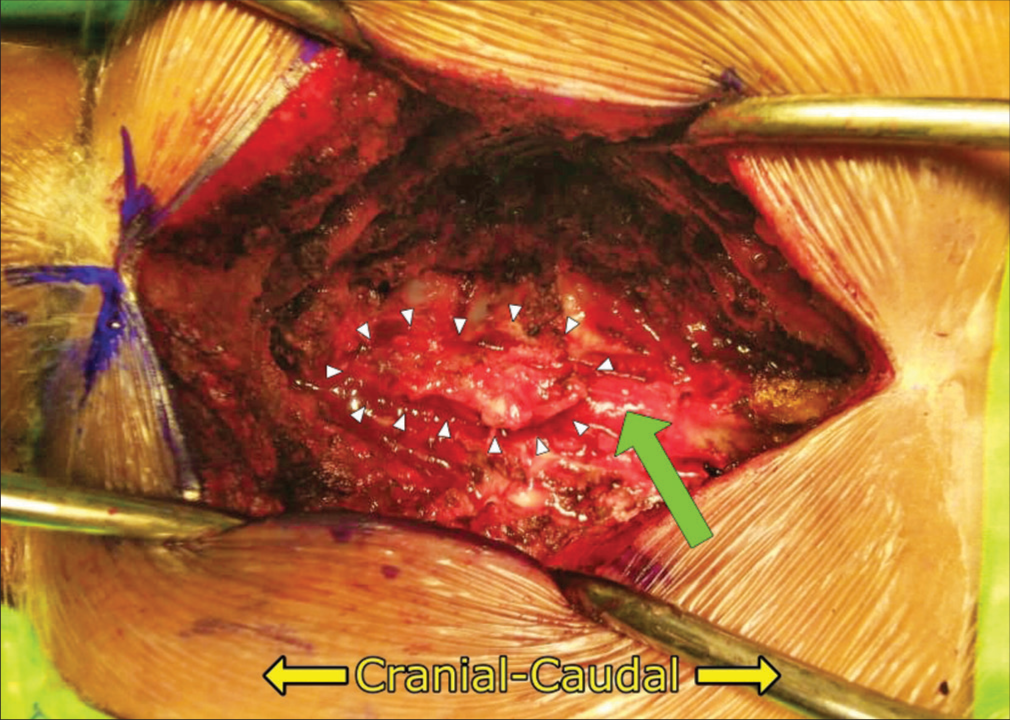
The pathology was consistent with the diagnosis of a Xanthogranuloma. The gross examination revealed brownish-white, soft-tissue fragments (i.e., largest measurements of 2.6 × 1.9 × 0.6 cm), while the microscopic showed fibrous connective tissue with extravasation of erythrocytes, xanthoma cells, lymphocytes, histiocytes, and Touton giant cells [
Postoperative course
On postoperative day 2, the patient’s lower extremity strength normalized while the sensation improved. He was discharged a few days later, was fully neurologically intact within 2 postoperative months, and continues to be followed with repeat MRI studies (i.e., every 6–12 months) that have remained negative for tumor recurrence.
DISCUSSION
Frequency
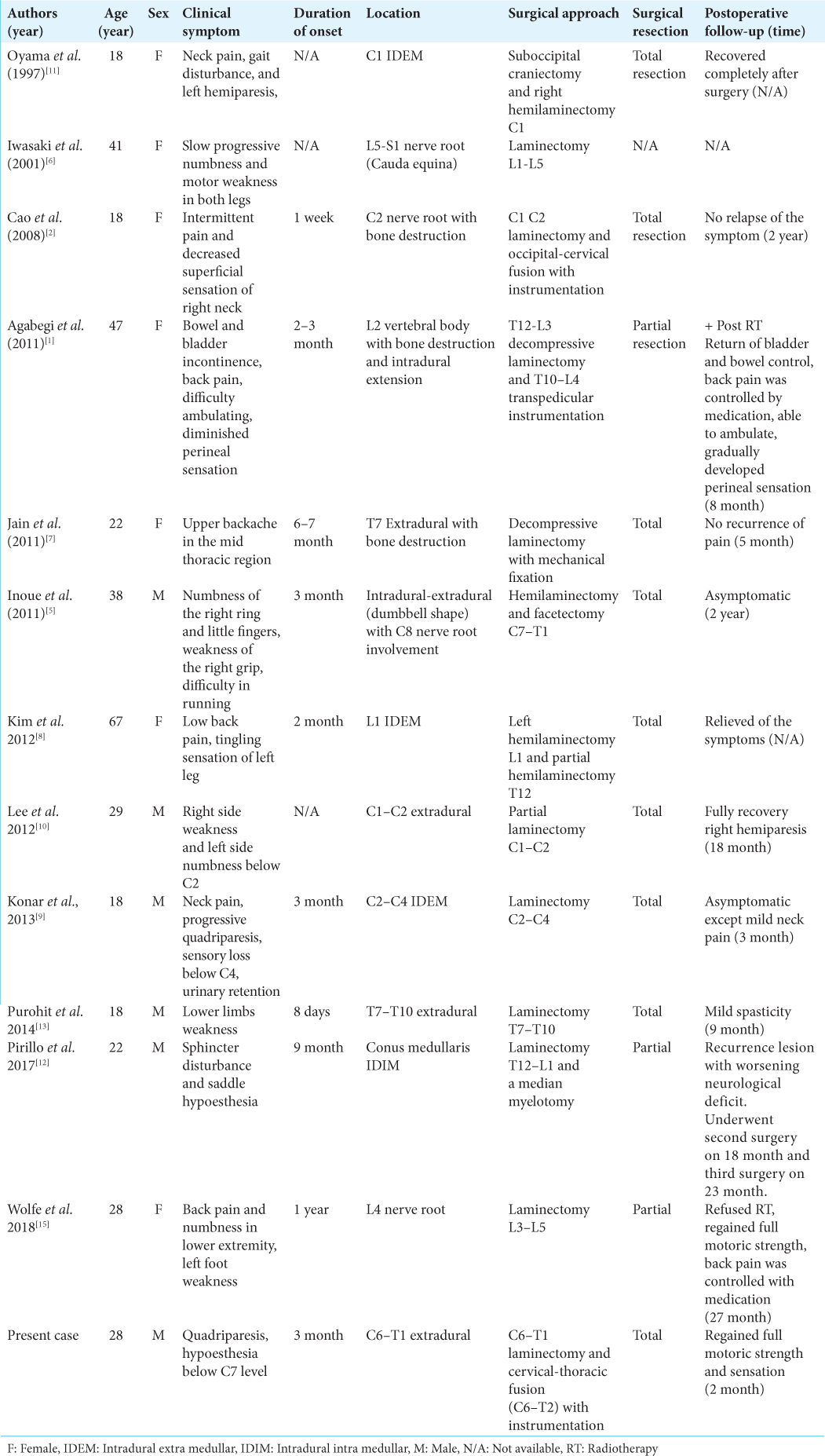
We combined our case with the 12 other cases of spinal JXG found in the literature [
Etiology of JXG
JXG is a histiocytic disorder of unknown etiology that belongs to the non-Langerhans dendritic cell disorders.[
Treatment option
Although gross total excision is the treatment of choice to attain cures for JXG, treatment options may be limited restricted by their location and/or adherence to surrounding critical structures.[
CONCLUSION
Spinal JXG in adults are extremely rate and should ideally be treated with gross total tumor excision to achieve the best long-term results.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agabegi SS, Iorio TE, Wilson JD, Fischgrund JS. Juvenile xanthogranuloma in an adult lumbar spine: A case report. Spine (Phila Pa 1976). 2011. 36: E69-73
2. Cao D, Ma J, Yang X, Xiao J. Solitary juvenile xanthogranuloma in the upper cervical spine: Case report and review of the literatures. Eur Spine J. 2008. 17: S318-23
3. Collie JS, Harper CD, Fillman EP, editors. Juvenile xanthogranuloma: In StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. p.
4. Dehner LP. Juvenile xanthogranulomas in the first two decades of life: A clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol. 2003. 27: 579-93
5. Inoue H, Seichi A, Yamamuro K, Kojima M, Kimura A, Hoshino Y. Dumbbell-type juvenile xanthogranuloma in the cervical spine of an adult. Eur Spine J. 2011. 20: S343-7
6. Iwasaki Y, Hida K, Nagashima K. Cauda equina xanthogranulomatosis. Br J Neurosurg. 2001. 15: 72-3
7. Jain A, Mathur K, Khatri S, Kasana S, Jain SK. Rare presentation of juvenile xanthogranuloma in the thoracic spine of an adult patient: Case report and literature review. Acta Neurochir (Wien). 2011. 153: 1813-8
8. Kim SY, Park HJ, Lee SY, Chung EC, Park HW, Kook SH. MRI findings of juvenile xanthogranuloma of the spinal cord: A case report. J Korean Soc Radiol. 2013. 68: 251
9. Konar S, Pandey P, Yasha TC. Solitary juvenile xanthogranuloma in cervical spine: Case report and review of literature. Turk Neurosurg. 2014. 24: 102-7
10. Lee SJ, Jo DJ, Lee SH, Kim SM. Solitary xanthogranuloma of the upper cervical spine in a male adult. J Korean Neurosurg Soc. 2012. 51: 54
11. Oyama H, Ikeda K, Inoue S, Katsumata T, Murakami S, Doi A. A case of intradural xanthogranuloma in the upper cervical spine. No Shinkei Geka. 1997. 25: 745-8
12. Pirillo V, Prontera A, Rizzo P, Cecchi PC, Maffei M, Schwarz A. A rare case of intramedullary solitary juvenile xanthogranuloma of the lumbar spine in the adult: A case report. J Spine Surg. 2017. 3: 504-8
13. Purohit D, Chanduka A, Sharma V, Mittal R, Singhvi S. Juvenile xanthogranuloma of adult spine: A rare case and review of literature. Asian J Neurosurg. 2014. 9: 239-9
14. Stover DG, Alapati S, Regueira O, Turner C, Whitlock JA. Treatment of juvenile xanthogranuloma. Pediatr Blood Cancer. 2008. 51: 130-3
15. Wolfe C, El Ahmadieh TY, Aoun SG, Vance AZ, Hatanpaa KJ, Wohlfeld B. Intradural juvenile xanthogranuloma with involvement of multiple nerve roots: A case report and review of the literature. World Neurosurg. 2018. 119: 189-96