- Department of Neurosurgery, Saga University, Saga, Japan.
DOI:10.25259/SNI_305_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kohei Inoue, Akihiko Momozaki, Takashi Furukawa, Fumitaka Yoshioka, Atsushi Ogata, Jun Masuoka, Tatsuya Abe. Case of de novo cerebral microbleeds in ischemic-type pediatric moyamoya disease. 14-Jun-2021;12:284
How to cite this URL: Kohei Inoue, Akihiko Momozaki, Takashi Furukawa, Fumitaka Yoshioka, Atsushi Ogata, Jun Masuoka, Tatsuya Abe. Case of de novo cerebral microbleeds in ischemic-type pediatric moyamoya disease. 14-Jun-2021;12:284. Available from: https://surgicalneurologyint.com/surgicalint-articles/10883/
Abstract
Background: Studies on pediatric patients with moyamoya disease who presented with de novo cerebral microbleeds (CMBs) are extremely rare.
Case Description: Herein, we report a 7-year-old boy with moyamoya disease who had de novo CMBs during treatment. He presented with transient left-side motor weakness and was diagnosed with moyamoya disease. He underwent revascularization surgery on the right cerebral hemisphere. Six months after the surgery, he presented with transient right-side motor weakness and MRA revealed progression of stenosis in the left middle cerebral artery. After another 3 months, three de novo CMBs were identified. He underwent revascularization surgery on the left side. The symptom disappeared completely after surgery and no additional de novo CMBs were identified 1 year after surgery.
Conclusion: This is the first report on de novo CMBs in pediatric patients. Although the significance of de novo CMBs in pediatric patients is completely unknown, attention should be paid to not only ischemic stroke but also hemorrhagic stroke. Although the short-term course is good in the current case, follow-up period is too short to assess for rebleeding and long-term follow-up is still important. Further, more cases should be collected.
Keywords: De novo cerebral microbleeds, Pediatric moyamoya disease, periventricular anastomosis
INTRODUCTION
Cerebral microbleeds (CMBs) are commonly detected on T2*-weighted imaging (T2*WI) and susceptibility-weighted imaging (SWI) in patients with moyamoya disease.[
CASE DESCRIPTION
A 7-year-old boy initially presented with transient left-side motor weakness and then visited the pediatric department of a local hospital. He was diagnosed with moyamoya disease based on magnetic resonance angiography (MRA) results [
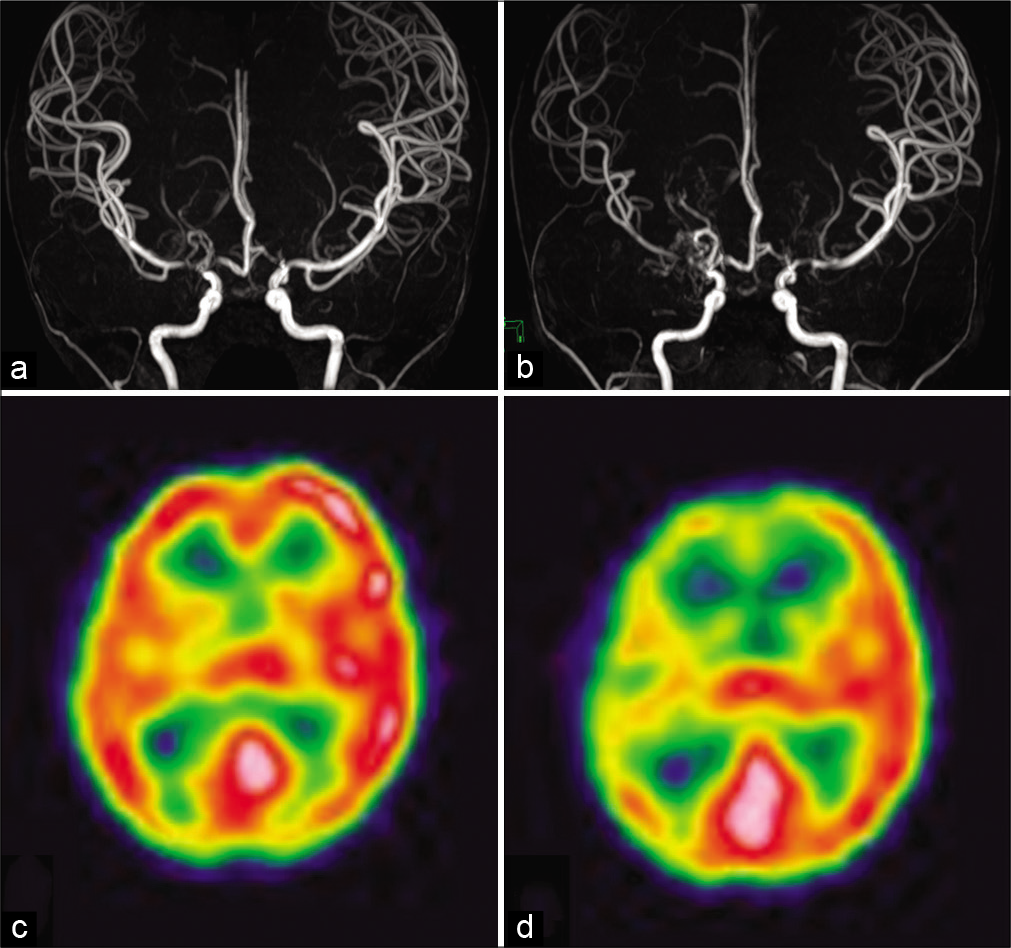
Figure 1:
(a) Initial magnetic resonance angiography (MRA) showed stenosis in the terminal portion of the bilateral internal carotid artery. (b) MRA performed after 1 year revealed progression of stenosis in the right middle cerebral artery. (c and d) Single-photon emission computed tomography with iodine-123 iodoamphetamine revealed preserved cerebral blood flow and a significant decrease in vascular reserve in the right hemisphere.
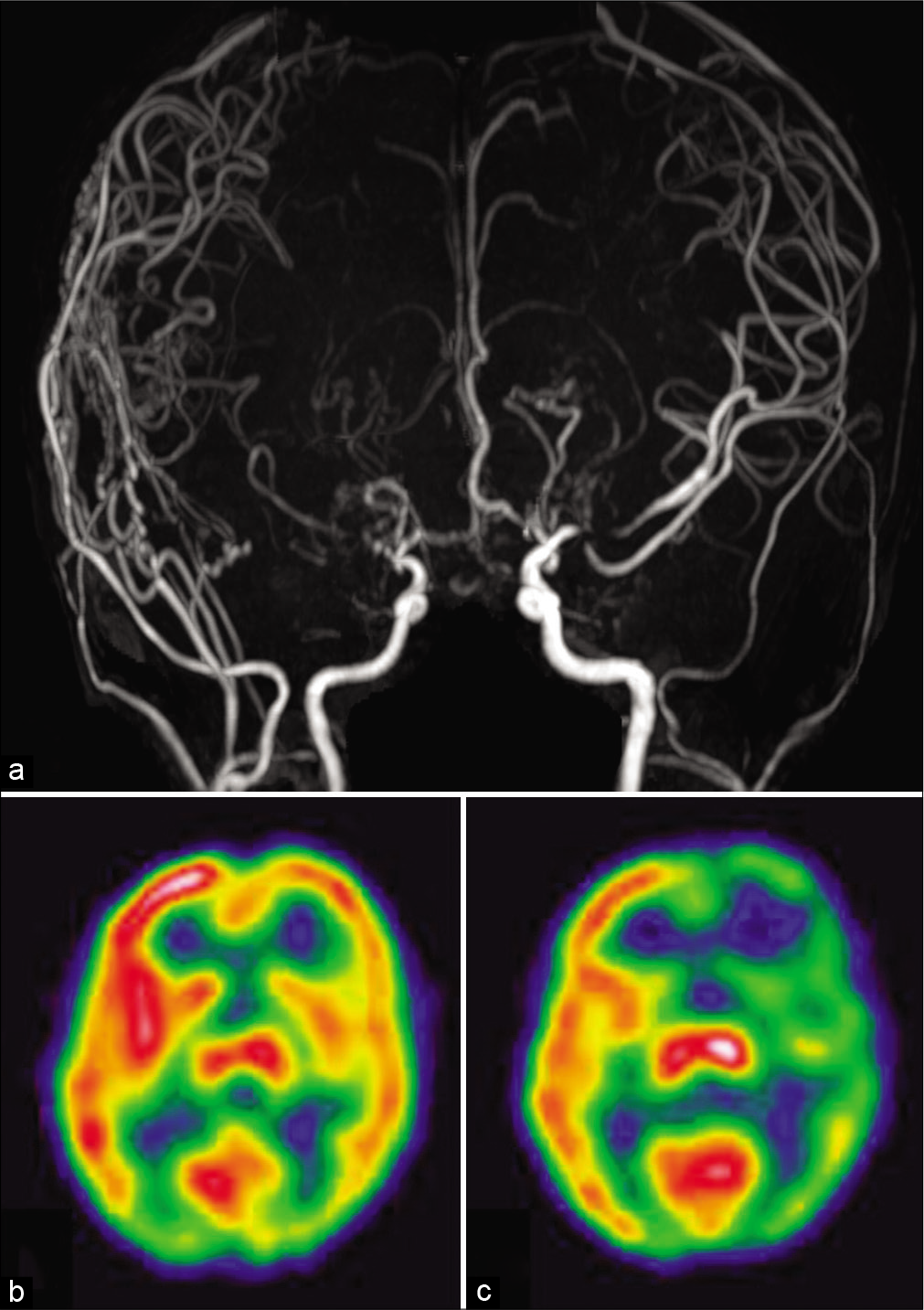
Figure 2:
(a) Magnetic resonance angiography revealed good angiogenesis from the external carotid system to the right cerebral hemisphere and progression of stenosis in the left middle cerebral artery. (b and c) Single-photon emission computed tomography with iodine-123 iodoamphetamine showed preserved cerebral blood flow and a significant decrease in vascular reserve in the left hemisphere.
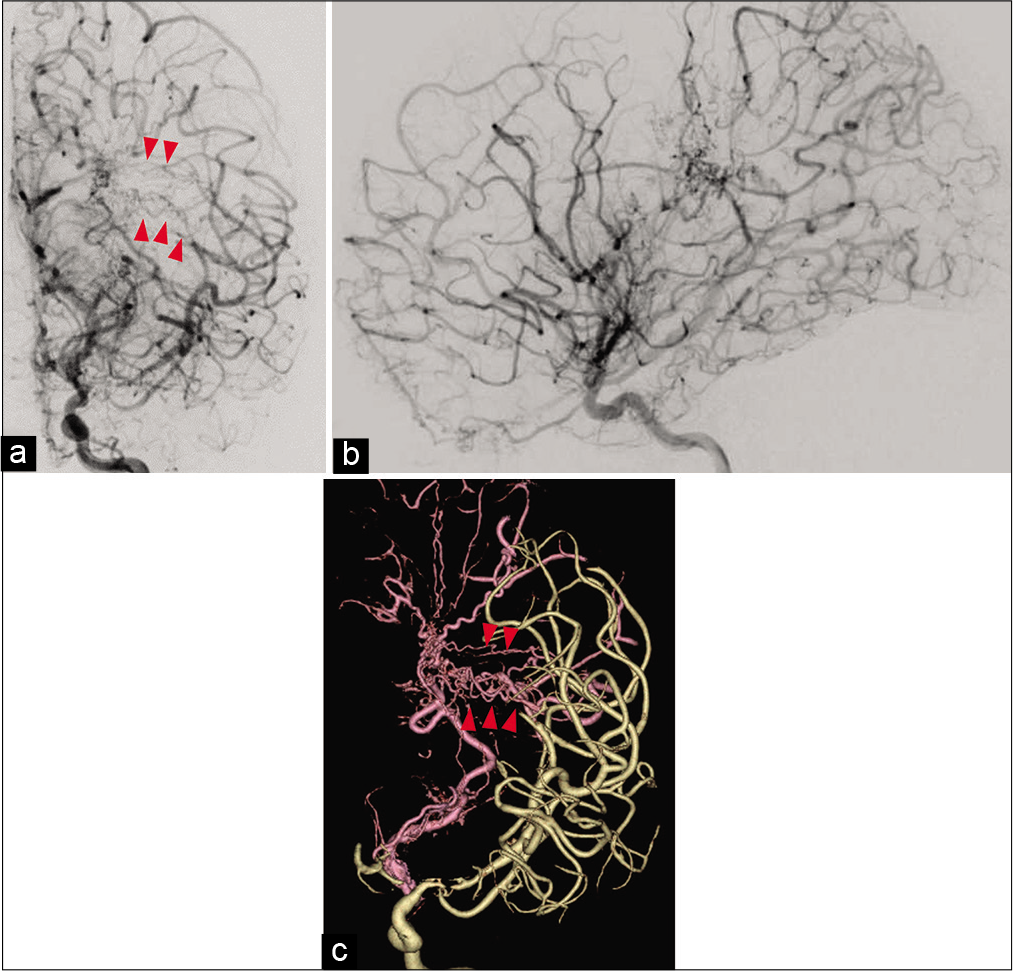
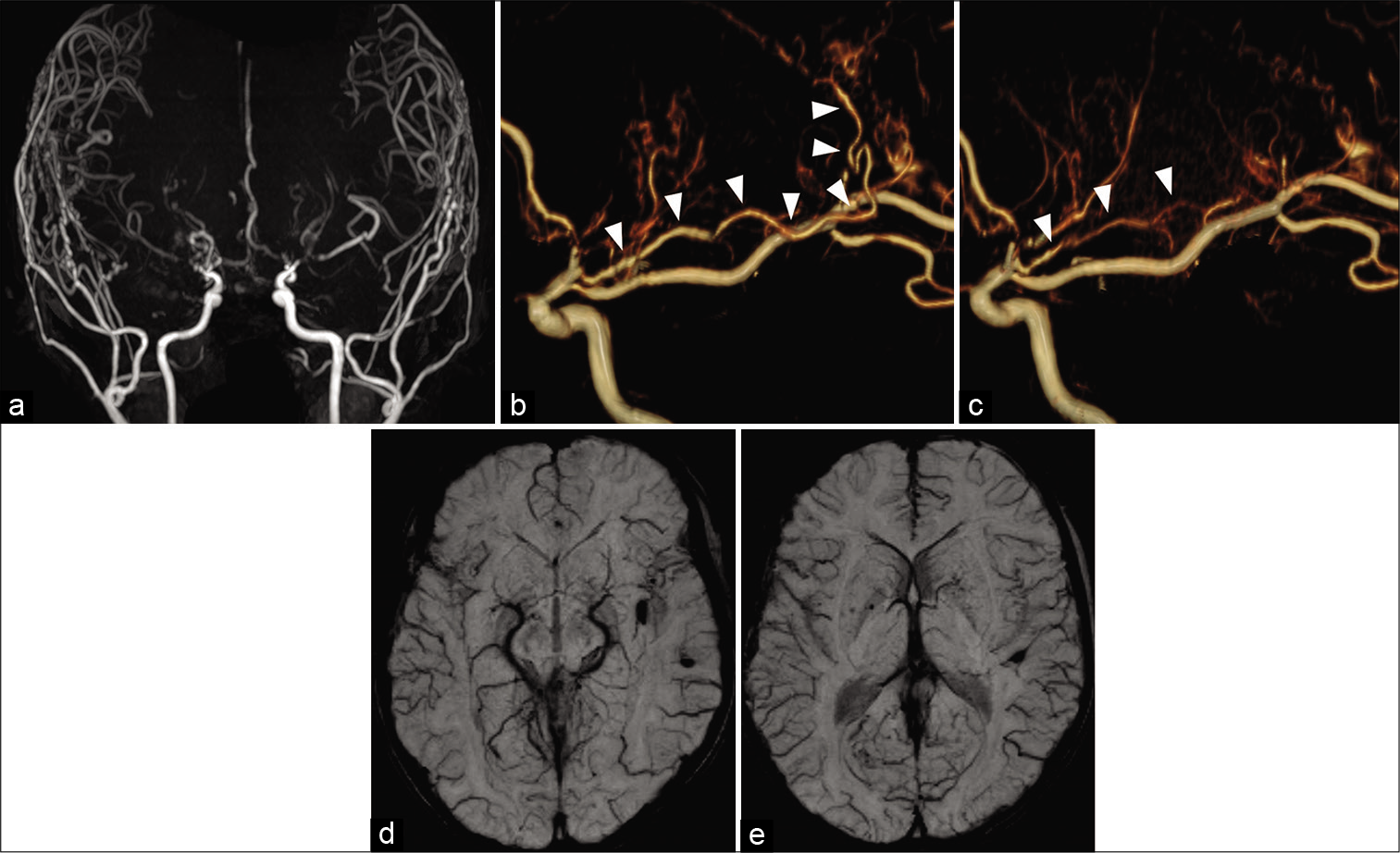
Figure 4:
Anteroposterior (a) and lateral (b) views on the left internal carotid angiography revealed the absence of cerebral aneurysm. Left internal carotid angiography (a) and three-dimension reconstruction angiography (c) revealed the abnormal collaterals from the anterior choroidal artery form periventricular anastomosis, which continued to the middle cerebral artery through the medullary artery (red arrowheads).
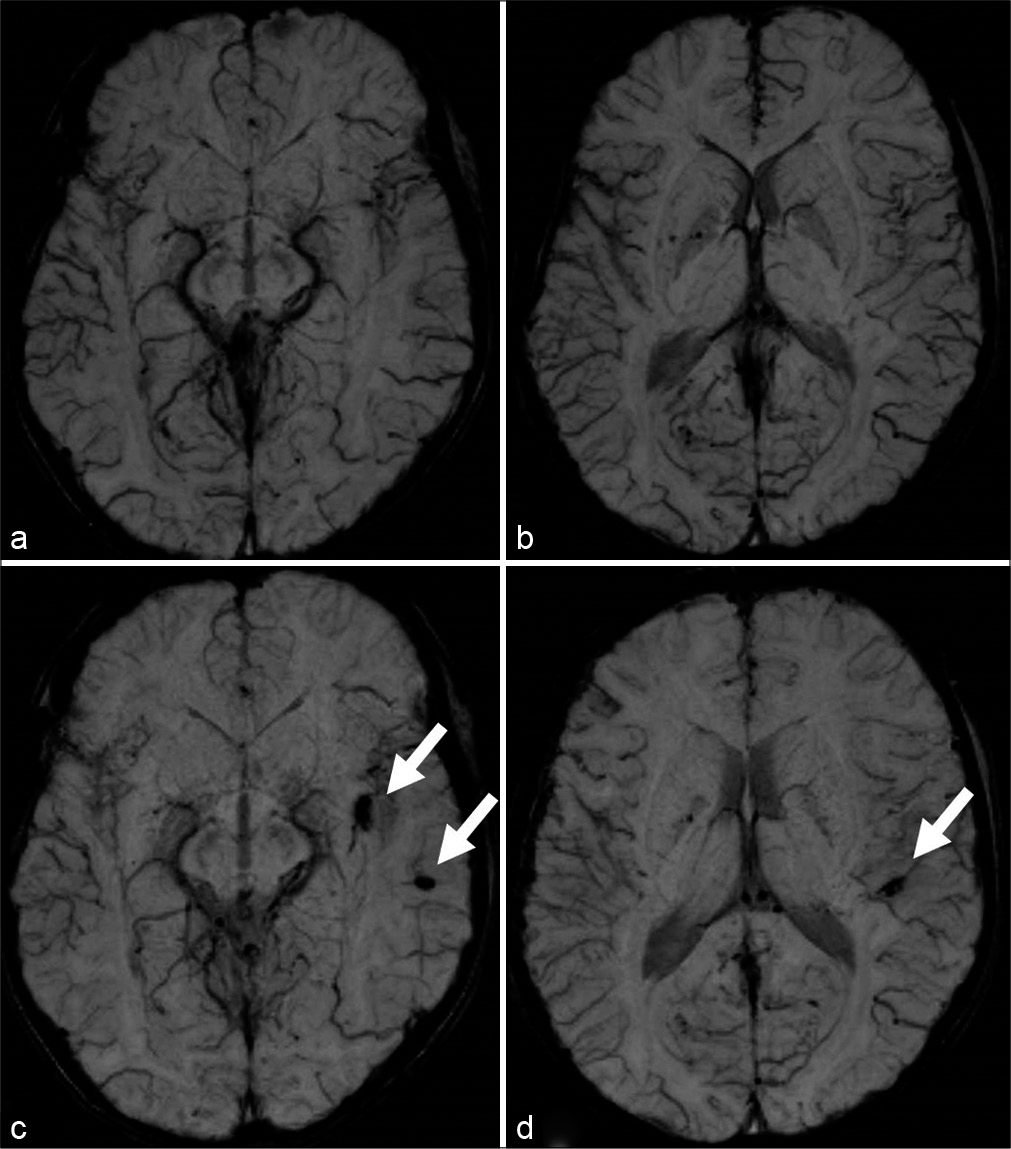
Figure 5:
(a) Magnetic resonance angiography (MRA) showed good angiogenesis from the external carotid system to the bilateral cerebral hemispheres. (b and c) Susceptibility-weighted imaging revealed no additional de novo cerebral microbleeds 1 year after surgery. Postoperative MRA (e) revealed shrinkage of the anterior choroidal artery and disappearance of abnormal collaterals (white arrowheads) compared to preoperative MRA (d).
DISCUSSION
In adult moyamoya disease, intracerebral and intraventricular hemorrhage is a major symptom, and it can be caused by the rupture of dilated and fragile moyamoya vessels caused by long-term hemodynamic stress. Recently, it has been shown that moyamoya vessels flow back into the medullary artery through periventricular anastomosis and perfuse the cerebral cortex, and vulnerability of periventricular anastomosis is considered to be the cause of hemorrhage. Funaki et al. classified the periventricular anastomosis into three types according to the perforating artery: lenticulostriate, thalamic, and choroidal type.[
All studies have included adult patients. Asymptomatic CMBs can be a significant predictor of hemorrhagic stroke in adult patients with moyamoya disease. To the best of our knowledge, asymptomatic CMBs are extremely rare in pediatric patients, and there are only two reports, seven available cases.[
CONCLUSION
Herein, we report a case of de novo CMBs in a pediatric patient with moyamoya disease. The significance of de novo CMB is unknown because it is an extremely rare condition. However, attention should be paid to not only ischemic stroke but also hemorrhagic stroke. Hence, more cases should be collected.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Enago for the English language review.
References
1. Ahn JH, Wang KC, Phi JH, Lee JY, Cho BK, Kim IO. Hemorrhagic moyamoya disease in children: Clinical features and surgical outcome. Childs Nerv Syst. 2012. 28: 237-45
2. Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008. 79: 900-4
3. Bao XY, Wang QN, Zhang Y, Zhang Q, Li DS, Yang WZ. Epidemiology of moyamoya disease in china: Single-center, population-based study. World Neurosurg. 2019. 122: e917-23
4. Chen PC, Yang SH, Chien KL, Tsai IJ, Kuo MF. Epidemiology of moyamoya disease in Taiwan: A nationwide population-based study. Stroke. 2014. 45: 1258-63
5. Funaki T, Takahashi JC, Houkin K, Kuroda S, Takeuchi S, Fujimura M. Angiographic features of hemorrhagic moyamoya disease with high recurrence risk: A supplementary analysis of the Japan adult moyamoya trial. J Neurosurg. 2018. 128: 777-84
6. Irikura K, Miyasaka Y, Kurata A, Tanaka R, Yamada M, Kan S. The effect of encephalo-myo-synangiosis on abnormal collateral vessels in childhood moyamoya disease. Neurol Res. 2000. 22: 341-6
7. Ishikawa T, Kuroda S, Nakayama N, Terae S, Kudou K, Iwasaki Y. Prevalence of asymptomatic microbleeds in patients with moyamoya disease. Neurol Med Chir (Tokyo). 2005. 45: 495-500
8. Jiang H, Ni W, Xu B, Lei Y, Tian Y, Xu F. Outcome in adult patients with hemorrhagic moyamoya disease after combined extracranial-intracranial bypass. J Neurosurg. 2014. 121: 1048-55
9. Kazumata K, Shinbo D, Ito M, Shichinohe H, Kuroda S, Nakayama N. Spatial relationship between cerebral microbleeds, moyamoya vessels, and hematoma in moyamoya disease. J Stroke Cerebrovasc Dis. 2014. 23: 1421-8
10. Kikuta K, Takagi Y, Nozaki K, Hanakawa T, Okada T, Mikuni N. Asymptomatic microbleeds in moyamoya disease: T2*-weighted gradient-echo magnetic resonance imaging study. J Neurosurg. 2005. 102: 470-5
11. Kikuta K, Takagi Y, Nozaki K, Okada T, Hashimoto N. Histological analysis of microbleed after surgical resection in a patient with moyamoya disease. Neurol Med Chir (Tokyo). 2007. 47: 564-7
12. Kikuta K, Takagi Y, Nozaki K, Sawamoto N, Fukuyama H, Hashimoto N. The presence of multiple microbleeds as a predictor of subsequent cerebral hemorrhage in patients with moyamoya disease. Neurosurgery. 2008. 62: 104-12
13. Kuroda S, Houkin K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008. 7: 1056-66
14. Kuroda S, Kashiwazaki D, Ishikawa T, Nakayama N, Houkin K. Incidence, locations, and longitudinal course of silent microbleeds in moyamoya disease: A prospective T2*-weighted MRI study. Stroke. 2013. 44: 516-8
15. Liu P, Han C, Li DS, Lv XL, Li YX, Duan L. Hemorrhagic moyamoya disease in children: Clinical, angiographic features, and long-term surgical outcome. Stroke. 2016. 47: 240-3
16. Mori N, Miki Y, Kikuta K, Fushimi Y, Okada T, Urayama S. Microbleeds in moyamoya disease: Susceptibility-weighted imaging versus T2*-weighted imaging at 3 Tesla. Invest Radiol. 2008. 43: 574-9
17. Qin Y, Ogawa T, Fujii S, Shinohara Y, Kitao S, Miyoshi F. High incidence of asymptomatic cerebral microbleeds in patients with hemorrhagic onset-type moyamoya disease: A phase-sensitive MRI study and meta-analysis. Acta Radiol. 2015. 56: 329-38
18. Sun W, Yuan C, Liu W, Li Y, Huang Z, Zhu W. Asymptomatic cerebral microbleeds in adult patients with moyamoya disease: A prospective cohort study with 2 years of follow-up. Cerebrovasc Dis. 2013. 35: 469-75
19. Wenz H, Wenz R, Maros M, Ehrlich G, Al-Zghloul M, Groden C. Incidence, locations, and longitudinal course of cerebral microbleeds in European moyamoya. Stroke. 2017. 48: 307-13
20. Yamamoto S, Kuroda S. Long-term effect of surgical revascularization on silent microbleeds in adult moyamoya disease: A case report. Surg Neurol Int. 2017. 8: 99
21. Yamao Y, Takahashi JC, Funaki T, Mineharu Y, Kikuchi T, Okada T. Revascularization surgery in childhood associated with a low incidence of microbleeds in adult patients with moyamoya. World Neurosurg. 2020. 133: e716-21