- Department of Neurosurgery, Universitas Airlangga - Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
Correspondence Address:
Nur Setiawan Suroto, Department of Neurosurgery, Universitas Airlangga -Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
DOI:10.25259/SNI_28_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nur Setiawan Suroto, Asra Al Fauzi, Ayu Yoniko Christi, Kevin Ariel Tiopan Simanjuntak, Perthdyatama Syifaq Budiono. Case of malignant brain edema despite successful recanalization after mechanical thrombectomy for anterior circulation stroke. 31-Mar-2023;14:111
How to cite this URL: Nur Setiawan Suroto, Asra Al Fauzi, Ayu Yoniko Christi, Kevin Ariel Tiopan Simanjuntak, Perthdyatama Syifaq Budiono. Case of malignant brain edema despite successful recanalization after mechanical thrombectomy for anterior circulation stroke. 31-Mar-2023;14:111. Available from: https://surgicalneurologyint.com/surgicalint-articles/12231/
Abstract
Background: Therapeutic reperfusion with endovascular treatment (EVT) for acute ischemic stroke is typically associated with better long-term functional outcome compared to standard medical care. However, post-procedural brain edema remained present in around half of EVT patients. Malignant brain edema (MBE) is a serious condition that can lead to increased intracranial pressure, rapid neurologic deterioration, and cerebral herniation, neutralizing the favorable efficacy of EVT on functional outcomes.
Case Description: A 51-year-old man with a history of atrial fibrillation presented with acute onset of hemiplegia and severe bradyarrhythmia. A head computed tomography-scan demonstrated hyperdense middle cerebral artery (MCA) sign. Intravenous thrombolysis was administered before temporary pacemaker insertion. The digital subtraction angiography confirmed occlusion of the M1 branch of the right MCA with no collaterals in the territory of the occluded vessel. Mechanical thrombectomy (MT) was performed 6 h after onset and successfully achieved modified thrombolysis in cerebral infarction 3 revascularization in 6 h 20 min. The patient later experienced massive brain edema that required emergent decompressive craniectomy. The modified Rankin scale score was 4 in 1- and 3-month’s follow-up.
Conclusion: MBE after MT results in unsatisfactory functional outcomes, even if it has successful revascularization. No collateral in the territory of the occluded vessel in the initial angiogram is one of the predictors of MBE after MT.
Keywords: Acute ischemic stroke, Endovascular treatment, Large vessel occlusion, Malignant brain edema, Mechanical thrombectomy
INTRODUCTION
In anterior circulation acute ischemic stroke (AIS), therapeutic reperfusion with endovascular treatment (EVT) is consistently linked to a favorable long-term functional prognosis over standard medical care.[
CASE PRESENTATION
Clinical history
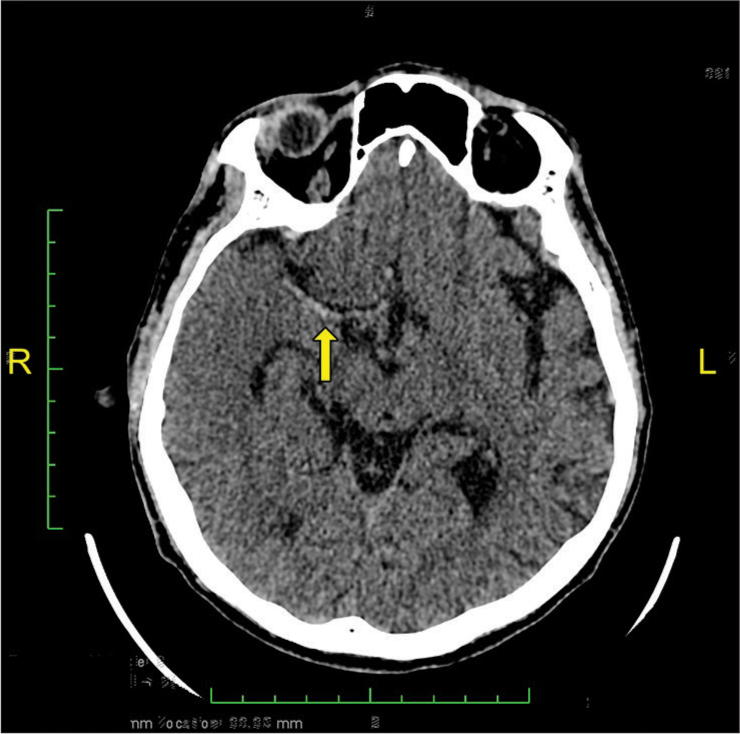
A 51-year-old man with a history of atrial fibrillation (AF) presented to the emergency department with acute onset of the left-sided weakness 45 min after his last known well (LNW). Further, anamnesis revealed that in the past 6th month, he did not take oral anticoagulation for his AF. The baseline National Institutes of Health Stroke Scale (NIHSS) score was 14, with left hemiplegia, right conjugate eye deviation, dysarthria, and facial paralysis. On initial examination, the patient was afebrile and alert with a Glasgow Coma Score (GCS) of 15, blood pressure was 140/67 mmHg, and his respiratory rate was 20 breaths/ min. In addition to that, his pulse was low, around 36 beats/ min. The electrocardiogram revealed a junctional rhythm. Sulfas atropine and dopamine were administered to treat the bradycardia since the pulse decreased to 20 beats/min and GCS dropped to 11 (E3V3M5). After hemodynamic stabilization, a head non-contrast computed tomography (NCCT) was performed (i.e., 2 h after LNW). A head NCCT revealed a hyperdense right middle cerebral artery (MCA) sign, suggesting a hyperacute thrombotic stroke [
EVT
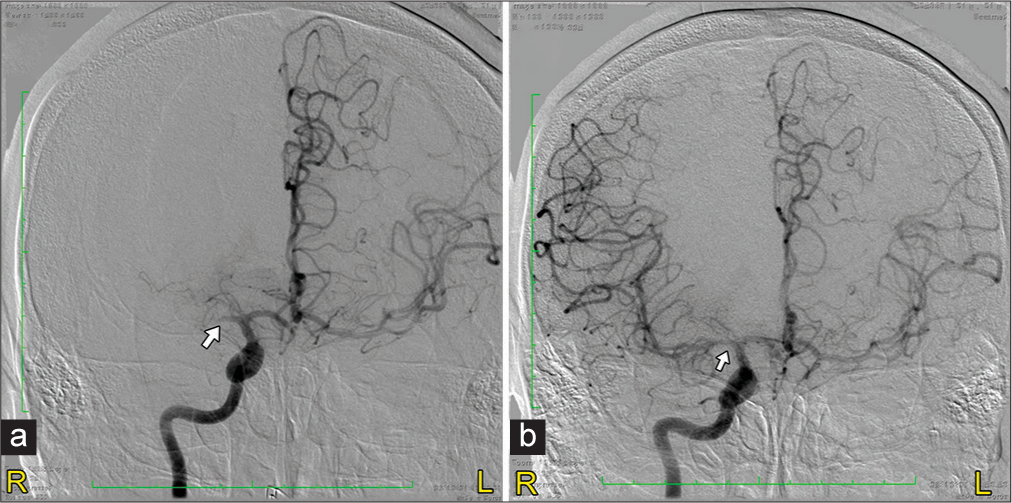
At 4 h after LNW, the patient received IVT using recombinant tissue plasminogen activator (alteplase) (r-tPA). Under General anesthesia, he was taken for temporary pacemaker (TPM) insertion by cardiologist, followed by EVT by our endovascular surgeon. An angiogram of the right internal carotid artery at hour 6 confirmed occlusion of the M1 branch of the right MCA with no collaterals in the territory of the occluded vessel [
Postoperative course
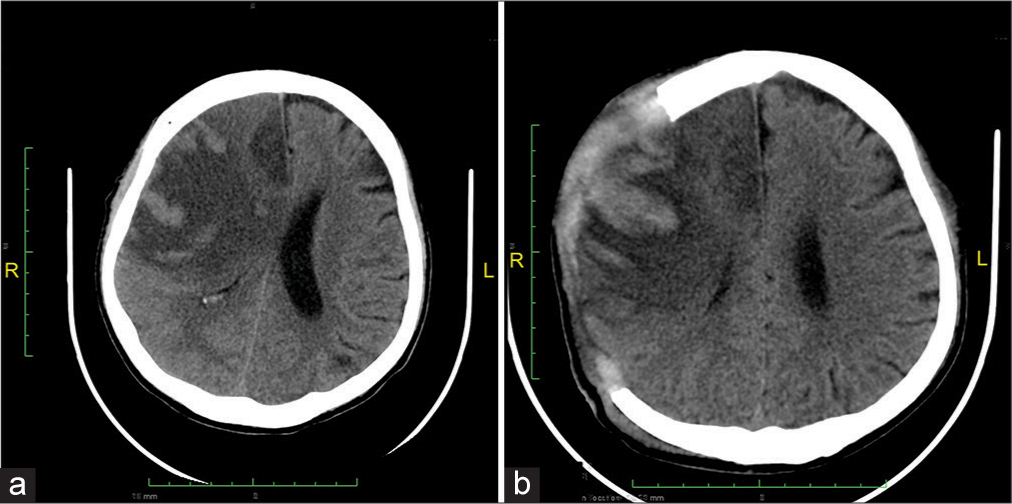
Post-thrombectomy, the patient was still intubated and sedated. During observation in the intensive care unit, he developed an occasional premature ventricular contraction and upside-down blood pressure. Follow-up NCCT [
The patient was treated conservatively initially for his brain edema. After being given conservative treatment for 2 days, the patient was not on sedation but still unconscious with GCS of E2V × M5. He developed anisocoria pupils with diameters of 2 mm and 1 mm, on the right and left pupils, respectively. The diagnosis of MBE was made, and the patient was taken for emergent decompressive craniectomy (DC) to release the intracranial pressure.
Two days following DC, the patient was allowed to awaken to the point where a limited neurologic examination was possible and subsequently extubated. He could move all four extremities on command but still had left hemiparesis, and his pupils were isochor. His NIHSS score was 6 for left hemiparesis and dysarthria. Follow-up NCCT was performed 4 days after DC [
DISCUSSION
In AIS, early restoration of blood flow to affected brain tissue has been proven to improve clinical outcomes. Therapeutic reperfusion with EVT is consistently linked to a favorable long-term functional prognosis over standard medical care.[
Nevertheless, a significant minority of patients do not achieve clinical improvement despite successful recanalization of the occluded artery and reperfusion of the infarcted area.[
In this case report, the patient had a history of AF but did not take anticoagulation therapy in the past 6 months. Thus, we decided to administer intravenous r-tPA while preparing the patient for urgent TPM insertion and EVT. According to American Heart Association/American Stroke Association (AHA/ASA) Guideline, patients eligible for intravenous r-tPA should receive intravenous r-tPA even if EVTs are being considered.[
MBE after an ischemic stroke is a serious condition that can lead to increased intracranial pressure, rapid neurologic deterioration, and cerebral herniation.[
Some studies[
Studies on the relationship between revascularization and brain edema have shown contradictory results. Pre-clinical rat and primate studies indicated that revascularization may lead to the development of brain edema.[
Huang et al. found that the presence of MBE in patients with successful recanalization after MT was associated with localization of occluded vessels (ICA occlusion vs. MCA occlusion: odds ratio [OR] = 3.746; 95% confidence interval [CI] 1.169–12.006; P = 0.026) and collateral score (grade 1 vs. grade 0: OR = 0.727; 95% CI 0.192–2.753; P = 0.638; grade 2 vs. grade 0: OR = 0.130; 95% CI 0.021–0.819; P = 0.030).[
Notably, Du et al. developed a nomogram that included collateral circulation to predict MBE in patients undergoing EVT.[
Collateral circulation plays a pivotal role in the pathophysiology of ischemic stroke.[
CONCLUSION
MBE after mechanical thrombectomy results in unsatisfactory functional outcomes, even if it has successful revascularization. No collateral in the territory of the occluded vessel in the initial angiogram is one of the predictors of MBE after MT. This case report described that there is a predictor factor of MBE that must be noted by the neurosurgeon regarding planning the management in the future and giving information to the patient’s family.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bell BA, Symon L, Branston NM. CBF and time thresholds for the formation of ischemic cerebral edema, and effect of reperfusion in baboons. J Neurosurg. 1985. 62: 31-41
2. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol. 2016. 15: 1138-47
3. Chen X, Huang Q, Deng Q, Shen R, Liu Y, Lu M. A prediction model of brain edema after endovascular treatment in patients with acute ischemic stroke. J Neurol Sci. 2019. 407: 116507
4. Du M, Huang X, Li S, Xu L, Yan B, Zhang Y. A nomogram model to predict malignant cerebral edema in ischemic stroke patients treated with endovascular thrombectomy: An observational study. Neuropsychiatr Dis Treat. 2020. 16: 2913-20
5. Elijovich L, Goyal N, Mainali S, Hoit D, Arthur AS, Whitehead M. CTA collateral score predicts infarct volume and clinical outcome after endovascular therapy for acute ischemic stroke: A retrospective chart review. J Neurointerv Surg. 2016. 8: 559-62
6. Gartshore G, Patterson J, Macrae IM. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp Neurol. 1997. 147: 353-60
7. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016. 387: 1723-31
8. Huang X, Yang Q, Shi X, Xu X, Ge L, Ding X. Predictors of malignant brain edema after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2019. 11: 994-8
9. Irvine HJ, Ostwaldt AC, Bevers MB, Dixon S, Battey TW, Campbell BC. Reperfusion after ischemic stroke is associated with reduced brain edema. J Cerebral Blood Flow Metab. 2018. 38: 1807-17
10. Kim JM, Bae JH, Park KY, Lee WJ, Byun JS, Ahn SW. Incidence and mechanism of early neurological deterioration after endovascular thrombectomy. J Neurol. 2019. 266: 609-15
11. Kimberly WT, Dutra BG, Boers AM, Alves HC, Berkhemer OA, van den Berg L. Association of reperfusion with brain edema in patients with acute ischemic stroke: A secondary analysis of the MR CLEAN Trial. JAMA Neurol. 2018. 75: 453-61
12. Liebeskind DS. Collateral circulation. Stroke. 2003. 34: 2279-84
13. Nogueira RG, Kemmling A, Souza LM, Payabvash S, Hirsch JA, Yoo AJ. Clinical diffusion mismatch better discriminates infarct growth than mean transit time-diffusion weighted imaging mismatch in patients with middle cerebral artery-M1 occlusion and limited infarct core. J Neurointerv Surg. 2017. 9: 127-30
14. Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G. Cerebral ischemia-reperfusion injury in rats-A 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009. 29: 1846-55
15. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC. 2015 American Heart Association/American stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American. Stroke. 2015. 46: 3020-35
16. Rabinstein AA, Albers GW, Brinjikji W, Koch S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int J Stroke. 2019. 14: 23-31
17. Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA. 2016. 316: 1279-88
18. Song SY, Ahn SY, Rhee JJ, Lee JW, Hur JW, Lee HK. Extent of contrast enhancement on non-enhanced computed tomography after intra-arterial thrombectomy for acute infarction on anterior circulation : As a predictive value for malignant brain edema. J Korean Neurosurg Soc. 2015. 58: 321-7
19. Zhang X, Yan S, Zhong W, Yu Y, Lou M. Early NT-ProBNP (N-terminal probrain natriuretic peptide) elevation predicts malignant edema and death after reperfusion therapy in acute ischemic stroke patients. Stroke. 2021. 52: 537-42