- Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, Maryland, United States.
Correspondence Address:
Joshua Olexa, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, Maryland, United States.
DOI:10.25259/SNI_856_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Joshua Olexa, Annie Trang, Rebecca Flessner, Mohammed Labib. Case report: Use of markerless augmented reality system for ventriculoperitoneal shunt placement. 29-Dec-2023;14:447
How to cite this URL: Joshua Olexa, Annie Trang, Rebecca Flessner, Mohammed Labib. Case report: Use of markerless augmented reality system for ventriculoperitoneal shunt placement. 29-Dec-2023;14:447. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12694
Abstract
Background: Ventriculoperitoneal (VP) shunt placement is one of the most commonly performed neurosurgical procedures, yet failure rates remain very high. Surface landmarks are typically used to guide VP shunt placement, but they are not reliable in identifying the target anatomy. Augmented reality (AR) is a promising new technology that has the potential to improve the accuracy and effectiveness of neurosurgical procedures. We describe the use of AR for the surgical planning of a VP shunt.
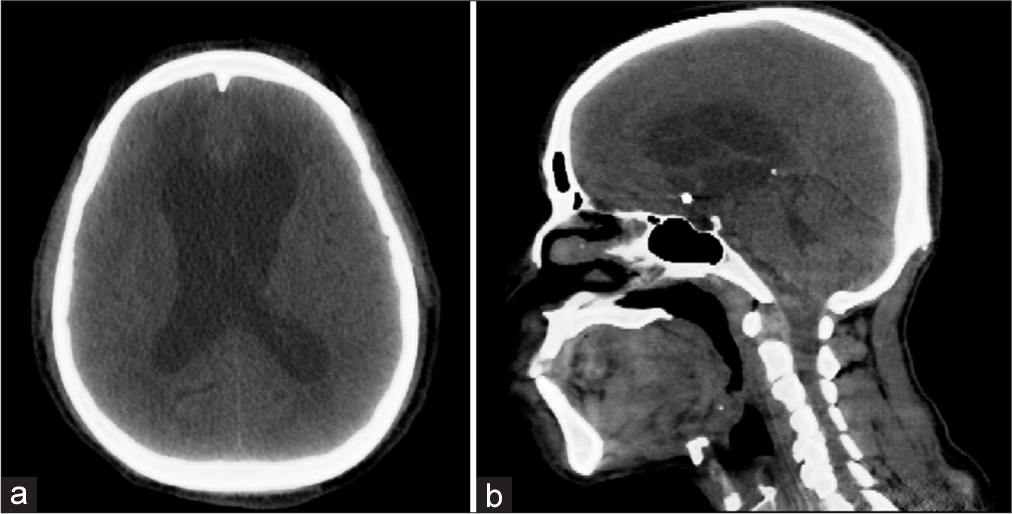
Case Description: A 62-year-old male with a history of subarachnoid hemorrhage presented with delayed hydrocephalus. A computed tomography scan was obtained that confirmed dilated ventricles, requiring a right VP shunt. The patient was brought to the operating room, where the AR system was used for visualization and planning.
Conclusion: In this study, we describe the use of AR for VP shunt placement. The AR system consists of a Microsoft HoloLens 2 head-mounted display and a novel markerless registration system, which was used to register patient-specific 3D models onto the patient’s head for visualizing target anatomy and planning an operative approach. The AR system was used to plan the VP shunt placement in the operating room. This system is easy to use and provides a visualization of the patient’s anatomy, which can be used to plan an optimal trajectory. We believe that this has the potential to improve the accuracy and outcomes of VP shunt placements, and further studies are needed to characterize the system’s accuracy and benefits.
Keywords: Augmented reality, Mixed reality, Presurgical planning, Ventriculoperitoneal shunt
INTRODUCTION
Insertion of a ventriculoperitoneal (VP) shunt is one of the most frequently performed neurosurgical procedures for the management of hydrocephalus. Although it is a frequently performed procedure, the failure rate of shunts is up to 39% in 1 year. The most common cause of failure is obstruction of the catheter.[
Augmented reality (AR) allows for 2D patient imaging, such as magnetic resonance imaging and computed tomography (CT), to be appreciated in 3D space. AR is a technology that can superimpose 3D virtual models onto the user’s real-world environment. This technology has the potential to improve the accuracy and effectiveness of surgical planning by providing surgeons with a 3D view of target anatomy and its surrounding structures that can be used to plan the ideal procedural approach. AR provides the benefits of both 3D visualization and the ability to visualize these models superimposed onto the real environment and patient. The purpose of this study is to assess the benefit of a novel AR registration system for operative planning of VP shunt placement.
CASE DESCRIPTION
A 62-year-old male with a history of subarachnoid hemorrhage presented with delayed hydrocephalus, requiring a right VP shunt. CT scan confirmed dilated ventricles [
Segmenting anatomical structures
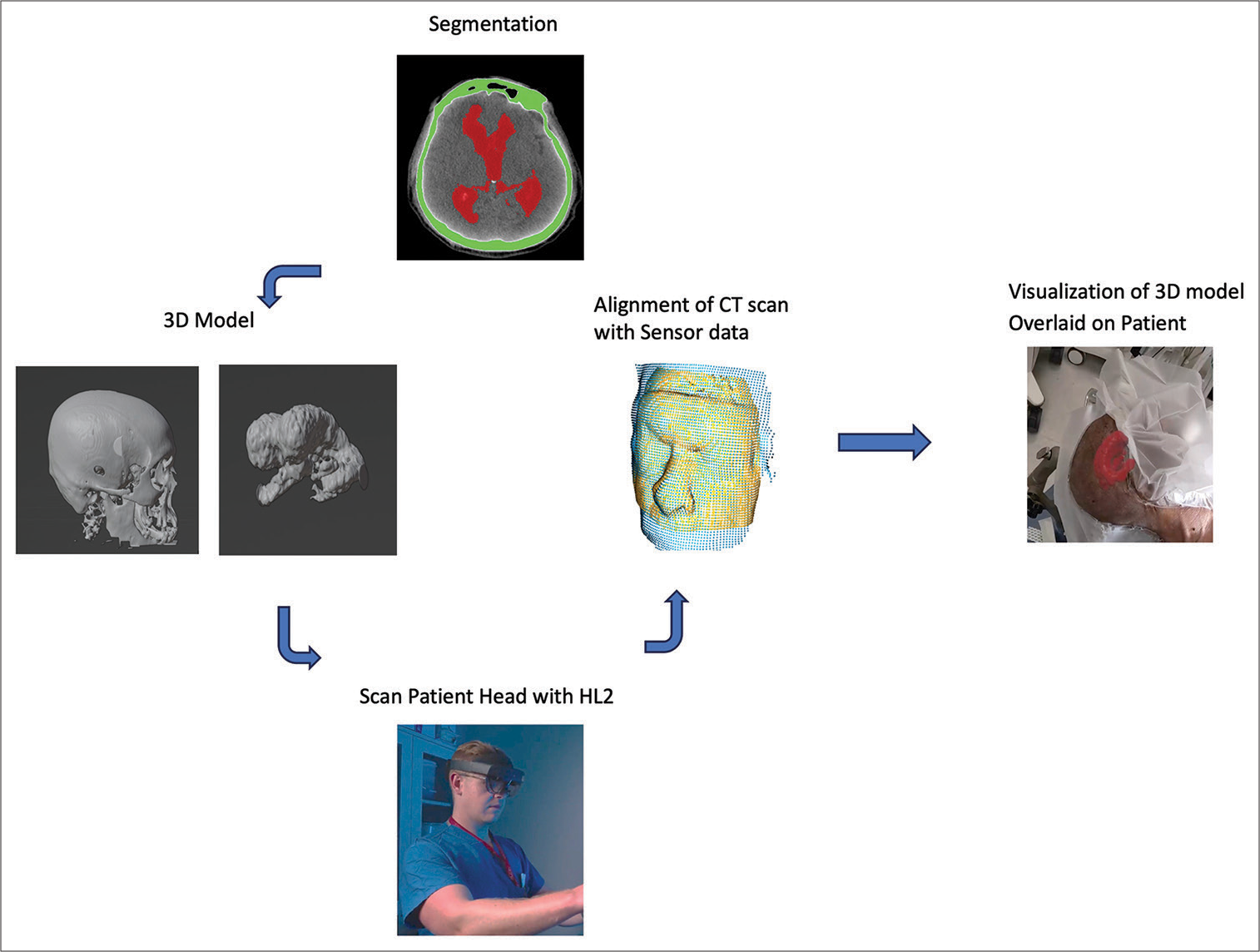
3D models were generated based on the patient’s preoperative CT scan that was obtained the morning of the procedure. Anatomy of interest – skull, and ventricles – were manually segmented using ITKsnap. The patient’s face was segmented and reconstructed for registration through auto-segmentation algorithms (Hoth Intelligence Inc., Philadelphia, Pennsylvania). All segmented structures were merged and converted into a 3D digital model [
Figure 2:
Workflow of the augmented reality (AR) technology platform. The patient’s anatomy is first segmented and converted into a 3D model. The model is loaded onto the Microsoft Hololens 2 (HL2) AR headset. While wearing the headset, the surgeon scans the patient’s head. Registration algorithms are processed on the headset, and the 3D model can be visualized aligned/overlaid with the patient’s head.
AR technology platform
The AR application was developed by Hoth Intelligence (Philadelphia, Pennsylvania) to operate on the Microsoft HoloLens 2 HMD (Redmond, Washington). The Microsoft HoloLens 2 is an untethered optical see-through HMD that superimposes virtual content (i.e., holograms, images, and screens) onto the users’ real-world field of view. A clinical workflow of the AR system is described in [
3D model registration to patient
The application used in this study superimposes 3D digital models onto the head using a proprietary ultra-fast, markerless registration process. To register the anatomy with the subject, the user looks at the subject’s head while wearing the HMD. Information from the headset sensors (depth sensor, RBG camera, and stereo sensor) are merged with coordinate data from the preoperative CT scan to align the 3D model to the patient’s head. After the patient was placed in the operative position, the patient-specific 3D model was registered onto the head through the AR headset [
Mixed reality viewing
Once registered, the physician was able to freely move around the patient to visualize the 3D model overlaid onto the patient’s head from different perspectives [
Video 1
Surgical strategy
Areas of the head, neck, and abdomen were prepped for incision. Dissection was carried down to the periosteum, and a burr hole was placed. Once the dura was coagulated and incised, neuronavigation was used once again to guide a catheter into the ventricle. The proximal catheter was connected to the valve and secured on the bone. The distal catheter was tunneled toward the right upper quadrant of the abdomen, connected to the reservoir, and tested. A right lower quadrant skin incision was made to introduce a trocar into the peritoneal cavity. Shunt tubing was then tunneled with the tip laying in the right lower quadrant. The galea and all skin incisions were then closed.
DISCUSSION
We describe a novel use of AR for use in presurgical planning for VP shunt placement. AR allows for integrating patient-specific anatomic information in 3D into the surgeon’s real-world environment without requiring the surgeon to look away from the patient and surgical field. We describe our experience using AR to overlay patient-specific 3D models onto the patient’s head with a markerless registration system. AR has emerged as a promising technology, gaining considerable traction in the field of neurosurgery with a range of applications in neuroanatomy education, surgical training, and intraoperative guidance.[
Image-guided surgery gives the surgeon the ability to visualize patient-specific anatomy to plan an appropriate approach and trajectory. Studies have shown improvements in catheter accuracy with image guidance.[
The AR system allowed the surgeon to visualize the patient’s abnormal target anatomy and to plan an appropriate catheter trajectory accordingly. The surgeons were able to visualize the 3D reconstructions of the patient’s anatomy. They were also able to plan and visualize virtual trajectories in real-time, although not at the time of burr-hole drilling and catheter insertion. This technology was used successfully in the operating room setting preoperatively, but this study has several limitations. This is a case study, and future work should include a larger number of cases to demonstrate improvements in placement accuracy as well as patient outcomes when using this AR visualization system. Future studies should include validation of the system’s registration accuracy and speed. The utility of the system across a variety of pathologies and user level of training should also be evaluated. The current system does not include instrument tracking in real-time. The ability to align surgical tools precisely with the trajectory and to determine when a surgical tool has reached a target structure could further improve the accuracy of catheter placement.
CONCLUSION
In this study, we report the use of AR visualization for planning VP shunt placement. This technology is advantageous in surgical planning as it allows for enhanced visualization with a fast, user-friendly setup. Future studies are needed to characterize the accuracy of this novel technology further, its impacts on surgical outcomes, and cost and usability in various clinical scenarios.
Ethical approval
Institutional Review Board approval is taken, approval number HP-00104849 Date: 10/22 University of Maryland Medical Center.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Durrani S, Onyedimma C, Jarrah R, Bhatti A, Nathani KR, Bhandarkar AR. The virtual vision of neurosurgery: How augmented reality and virtual reality are transforming the neurosurgical operating room. World Neurosurg. 2022. 168: 190-201
2. Gilard V, Magne N, Gerardin E, Curey S, Pelletier V, Hannequin P. Comparison of electromagnetic neuronavigation system and freehand method for ventricular catheter placement in internal shunt. Clin Neurol Neurosurg. 2017. 158: 93-7
3. Jung N, Kim D. Effect of electromagnetic navigated ventriculoperitoneal shunt placement on failure rates. J Korean Neurosurg Soc. 2013. 53: 150-4
4. Lind CR, Tsai AM, Law AJ, Lau H, Muthiah K. Ventricular catheter trajectories from traditional shunt approaches: A morphometric study in adults with hydrocephalus. J Neurosurg. 2008. 108: 930-3
5. Olexa J, Cohen J, Alexander T, Brown C, Schwartzbauer G, Woodworth GF. Expanding educational frontiers in neurosurgery: Current and future uses of augmented reality. Neurosurgery. 2023. 92: 241-50
6. Reddy GK, Bollam P, Shi R, Guthikonda B, Nanda A. Management of adult hydrocephalus with ventriculoperitoneal shunts: Long-term single-institution experience. Neurosurgery. 2011. 69: 774-80
7. Wilson TJ, Stetler WR, Al-Holou WN, Sullivan SE. Comparison of the accuracy of ventricular catheter placement using freehand placement, ultrasonic guidance, and stereotactic neuronavigation. J Neurosurg. 2013. 119: 66-70
8. Yamada S, Ishikawa M, Nakajima M, Nozaki K. Reconsidering ventriculoperitoneal shunt surgery and postoperative shunt valve pressure adjustment: Our approaches learned from past challenges and failures. 2021. 12: 798488