- Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Ashok Kumar Mahapatra
Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India
DOI:10.4103/2152-7806.166167
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prasad GL, Mahapatra AK. Case series of choroid plexus papilloma in children at uncommon locations and review of the literature. Surg Neurol Int 28-Sep-2015;6:151
How to cite this URL: Prasad GL, Mahapatra AK. Case series of choroid plexus papilloma in children at uncommon locations and review of the literature. Surg Neurol Int 28-Sep-2015;6:151. Available from: http://surgicalneurologyint.com/surgicalint_articles/case-series-of-choroid-plexus-papilloma-in-children-at-uncommon/
Abstract
Background:Choroid plexus papillomas (CPPs) comprise around 1% of intracranial neoplasms. The most common location is atrium of the lateral ventricle in children and fourth ventricle in adults. Other rare locations include third ventricle, cerebellopontine (CP) angle and cerebral parenchyma, with only a few cases reported. Authors report three cases of CPP at uncommon locations in pediatric patients. The rarity of these locations, diagnostic dilemma and management aspects are discussed along with an extensive review of the literature.
Methods:Retrospective institutional data analysis of histopathologically confirmed pediatric CPPs from 2010 to 2014.
Results:Authors noted three cases of CPP in children in uncommon locations-one each in the posterior third ventricle, fourth ventricle, and CP angle. All were males in the first decade. Two cases presented with features of obstructive hydrocephalus while the latter presented with compressive effects. Complete excision was achieved in two cases while subtotal removal was performed in one case (fourth ventricular) because of excess blood loss. Mean follow-up duration was 24.6 months (range 20–30 months). One case (of subtotal removal) had fair recovery while other two had excellent outcomes.
Conclusions:Posterior third ventricle, fourth ventricle, and CP angle are uncommon locations for these tumors in children. Complete surgical removal is the treatment of choice and approach needs to be tailored according to the site and size of the lesion. Blood loss is a major concern in young children as they are highly vascular tumors. Complete removal leads to excellent long-term survival rates. Adjuvant treatment is not required.
Keywords: Cerebellopontine angle, choroid plexus papilloma, fourth ventricle, pediatric, posterior third ventricle
INTRODUCTION
Choroid plexus papillomas (CPPs) are uncommon tumors of the central nervous system arising from the choroid plexus. They constitute around 0.5–1% in adults and 3–4% in children.[
METHODS
Authors conducted a retrospective data analysis of histologically confirmed CPPs in patients aged ≤16 years operated at our institute from 2010 to 2014 after obtaining Institutional Ethics Committee approval. Three cases were found to be present in rare locations one each in the posterior third ventricle, fourth ventricle, and CP angle. The clinico-radiological features, surgical details, and outcome were analyzed in detail. Furthermore, a review of literature is included.
CASE DESCRIPTIONS
Case 1
A 5-year-old boy presented with complaints of headache and vomiting of 3 months duration. Neurological examination revealed papilledema and mild upgaze paresis. Noncontrast computed tomography (CT) showed an isodense frond-like lesion in the posterior third ventricle with hydrocephalus [
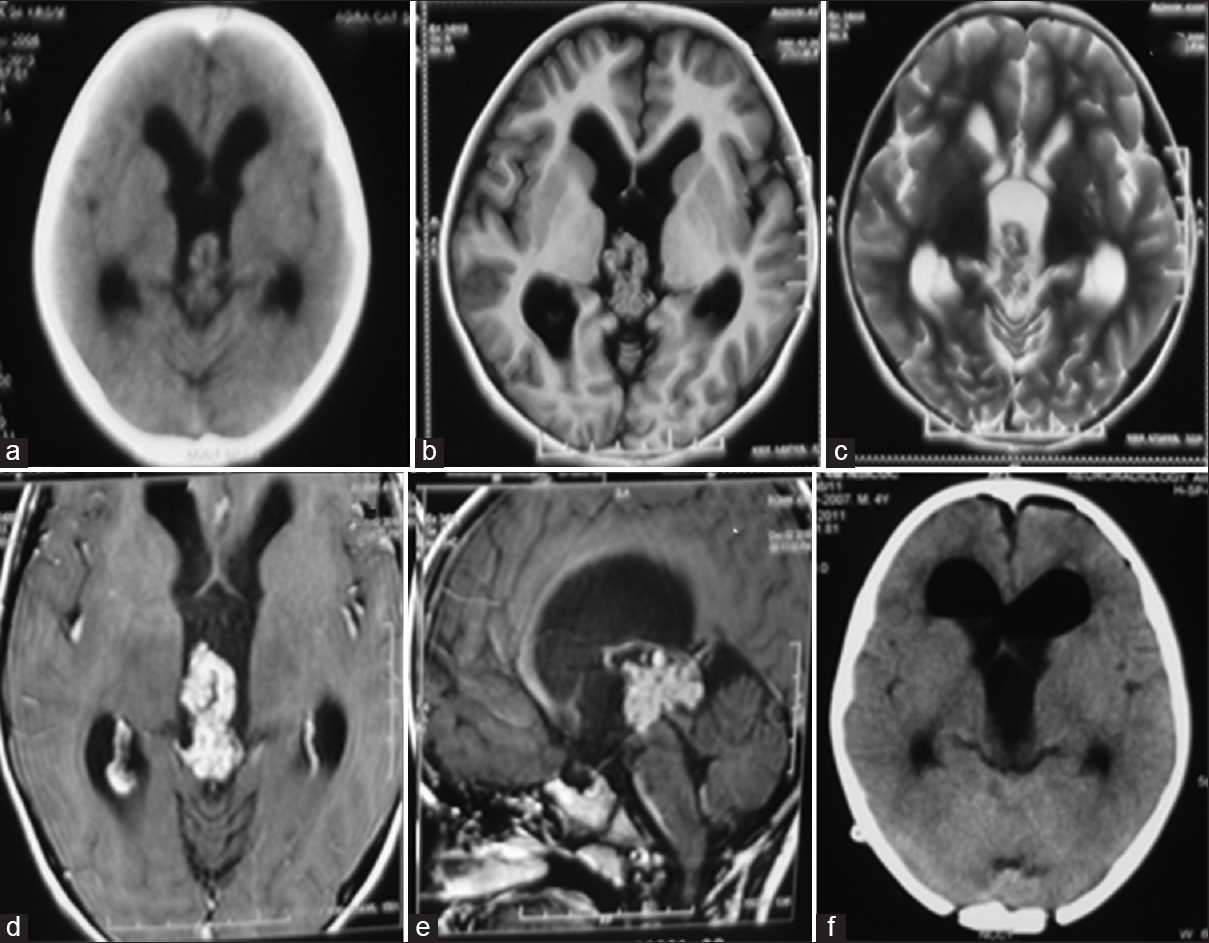
Figure 1
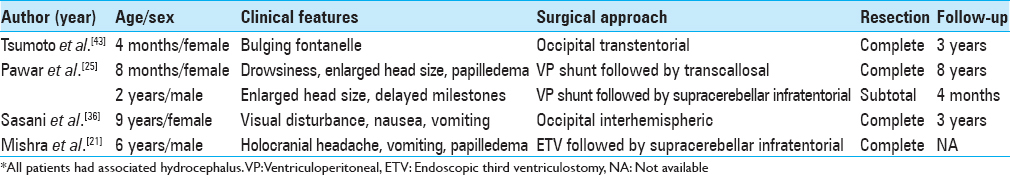
Case 1. Noncontrast computed tomography showing an isodense lobulated lesion in the posterior third ventricle with obstructive hydrocephalus (a). Plain magnetic resonance imaging axial sections showing the lobulated tumor appearing isointense on T1 and slightly hypointense on T2 sequences (b and c). Axial and sagittal postcontrast magnetic resonance images showing intense, homogeneous enhancement of the lesion, and located caudal to the deep venous system (d and e). Postoperative computed tomography showing no residual tumor with pneumoventricle seen in bilateral frontal horns (f)
Histopathology showed a papillary tumor lined by columnar epithelium with centrally placed nuclei. There was no mitosis or necrosis [
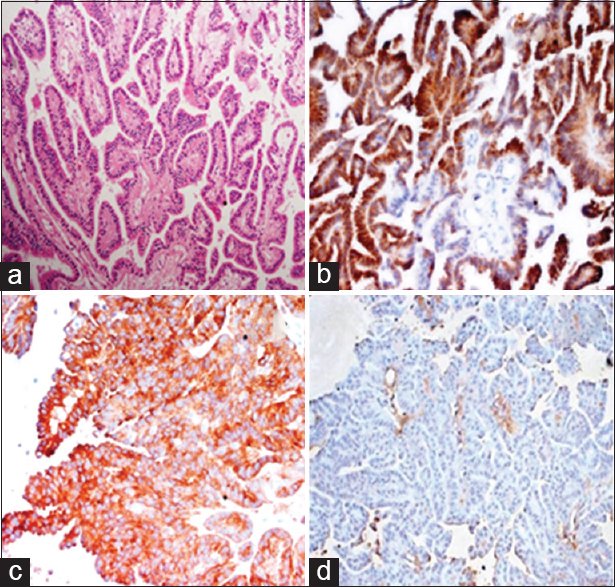
Figure 2
Histopathological images showing a papillary tumor with smooth papillae lined by single layer of columnar cells with no mitosis (a). Immunohistochemical images staining for pancytokeratin and synaptophysin were positive (b and c) while being negative for epithelial membrane antigen (d). Features were compatible with choroid plexus papilloma
Case 2
A 5-year-old boy presented with gait ataxia of 7 months duration and 2 months of headache and nasal regurgitation. Child was drowsy, but following commands; had a visual acuity of finger counting close to face, papilledema, and impaired gag reflex bilaterally. A noncontrast CT head showed a hyperdense midline posterior fossa mass lesion involving the fourth ventricle extending into the left foramen of Lushka with obstructive hydrocephalus. On MRI, the lesion was isointense on T1 and heterogeneously hyperintense on T2 sequences, showed intense enhancement and measured 4.8 cm × 3.5 cm × 5.2 cm [Figure
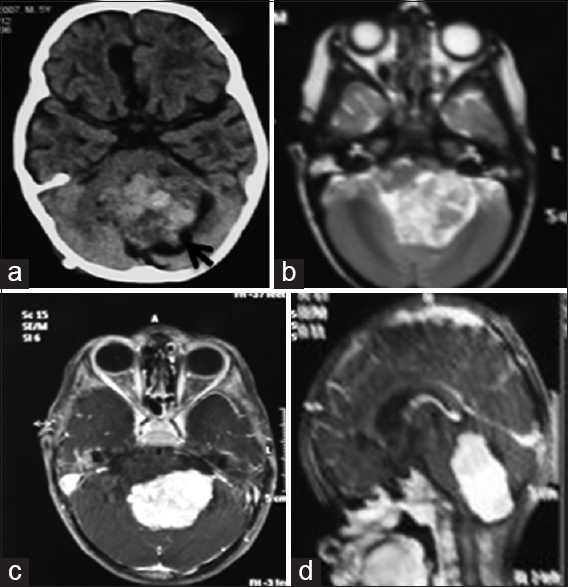
Figure 3
Case 2. Noncontrast computed tomography showing a heterogeneously hyperdense lesion in the midline posterior fossa with small peripheral cysts (black arrow) (a). A mild extension to the left cerebellopontine angle can be noted. Axial T2-weighted magnetic resonance images showing the lesion to be heterogeneously hyperintense (b). Axial and sagittal postcontrast magnetic resonance images showing intense, homogeneous enhancement of the lesion (c and d)
Case 3
An 8-year-old boy presented with 5 months duration of headache, vomiting, facial asymmetry, gait imbalance, and left-sided hearing loss. Neurological examination revealed bilateral papilledema, left sided abducent nerve paresis, Grade 3 LMN facial palsy, sensorineural hearing loss, and cerebellar signs. On MRI, a well-defined lobulated extra-axial left CP angle lesion was noted, appearing isointense, and heterogeneously hyperintense on T1 and T2 sequences respectively with moderate hydrocephalus and showing intense homogenous enhancement [Figure
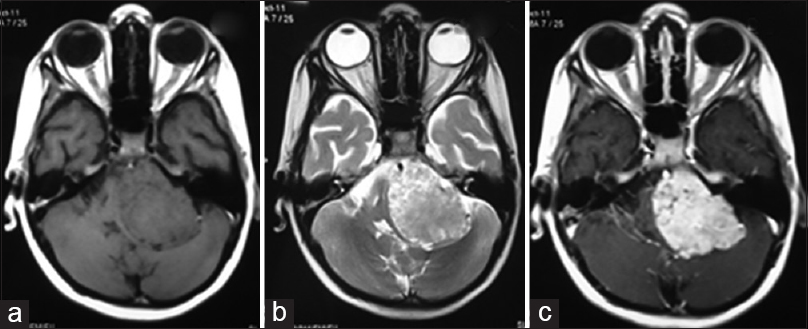
Figure 4
Case 3. Axial plain magnetic resonance imaging showing a left sided extra-axial cerebellopontine angle lesion which appears isointense and heterogeneously hyperintense on T1 and T2 sequences, respectively, with compression and deformation of the brainstem (a and b). Axial postcontrast magnetic resonance images showing homogeneous enhancement of the lesion (c)
DISCUSSION
CPPs are benign, uncommon neoplasms of the central nervous system. Derived from the neuroectoderm and arising from the choroid plexus lining the ventricles, overproduction of cerebrospinal fluid (CSF) is the hallmark of these tumors.[
Clinical features
Guerard, in 1832 described the first case of CPP.[
Imaging
On CT, most lesions appear as isodense/hyperdense well-defined lobulated mass with intense homogeneous enhancement.[
Pathology
Grossly, they are capsulated, soft, reddish-pink, and friable tumors with a cauliflower-like appearance. Microscopically, they resemble normal choroid plexus, albeit with minimal/no atypia. These tumors show strong positivity for cytokeratin (CK 7) due to their neuroectodermal origin. All cases express vimentin, S-100 protein, and synaptophysin. There might be focal reactivity for glial fibrillary acid protein (GFAP) and EMA; however, CK 20 and carcino-embryonic antigen are negative.[
Treatment
Surgical removal is the treatment of choice, and gross total removal should be attempted in all cases.[
CONCLUSIONS
CPPs are uncommon, benign, primary intracranial tumors. Posterior third ventricle, fourth ventricle, and CP angle are uncommon locations for these tumors in children. Raised ICP is the most common manifestation. They should always be considered in the differential diagnosis in children in any location, in the presence of supportive imaging findings. Surgical removal is the treatment of choice and approaches need to be tailored according to location, size, and extent of the tumor. These tumors are highly vascular, a feature which must be taken into consideration during surgery, especially in young children. Early securing of the arterial feeder is essential to reduce blood loss. Complete resection is the standard of care, and the long-term survival rate is excellent after complete removal. No adjuvant treatment is required, except in atypical and aggressive cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors would like to thank Dr. Kiran Subbarao for supporting with the histopathological images.
References
1. Acharya R, Bhutani A, Vasdev N, Madan VS. Choroid plexus papilloma of the fourth ventricle. Indian J Pathol Microbiol. 2002. 45: 103-5
2. Aguzzi A, Brandner S, Paulus W, Kleihues P, Cavenee W.editors. Choroid plexus tumours. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: IARC; 2000. p. 84-6
3. Bettegowda C, Adogwa O, Mehta V, Chaichana KL, Weingart J, Carson BS. Treatment of choroid plexus tumors: A 20-year single institutional experience. J Neurosurg Pediatr. 2012. 10: 398-405
4. Coates TL, Hinshaw DB, Peckman N, Thompson JR, Hasso AN, Holshouser BA. Pediatric choroid plexus neoplasms: MR, CT, and pathologic correlation. Radiology. 1989. 173: 81-8
5. Davis LE, Cushing H. Papillomas of the choroid plexus with report of six cases. Arch Neurol Psychiatry. 1925. 13: 681-710
6. Do HM, Marx WF, Khanam H, Jensen ME. Choroid plexus papilloma of the third ventricle: Angiography, preoperative embolization, and histology. Neuroradiology. 2001. 43: 503-6
7. Due-Tønnessen B, Helseth E, Skullerud K, Lundar T. Choroid plexus tumors in children and young adults: Report of 16 consecutive cases. Childs Nerv Syst. 2001. 17: 252-6
8. Duke BJ, Kindt GW, Breeze RE. Pineal region choroid plexus papilloma treated with stereotactic radiosurgery: A case study. Comput Aided Surg. 1997. 2: 135-8
9. Fujio S, Hirano H, Kawano H, Hanaya R, Oyoshi T, Niiro M. Three pediatric cases with choroid plexus papilloma in the fourth ventricle. No Shinkei Geka. 2010. 38: 149-55
10. Greene RC. Extraventricular and intra-cerebellar papilloma of the choroid plexus. J Neuropathol Exp Neurol. 1951. 10: 204-7
11. Hammock MK, Milhorat TH, Breckbill DL. Primary choroid plexus papilloma of the cerebellopontine angle presenting as brain stem tumor in child. Childs Brain. 1976. 2: 132-42
12. Jaiswal AK, Jaiswal S, Sahu RN, Das KB, Jain VK, Behari S. Choroid plexus papilloma in children: Diagnostic and surgical considerations. J Pediatr Neurosci. 2009. 4: 10-6
13. Kamar FG, Kairouz VF, Nasser SM, Faddoul SG, Saikali IC. Atypical choroid plexus papilloma treated with single agent bevacizumab. Rare Tumors. 2014. 6: 4687-
14. Kim IY, Niranjan A, Kondziolka D, Flickinger JC, Lunsford LD. Gamma knife radiosurgery for treatment resistant choroid plexus papillomas. J Neurooncol. 2008. 90: 105-10
15. Koeller KK, Sandberg GD. From the archives of the AFIP. Cerebral intraventricular neoplasms: Radiologic-pathologic correlation. Radiographics. 2002. 22: 1473-505
16. Kroppenstedt SN, Golfinos J, Sonntag VK, Spetzler RF. Pineal region lesion masquerading choroid plexus papilloma: Case report. Surg Neurol. 2003. 59: 124-7
17. Kumar R, Achari G, Benerji D, Jain VK, Chhabra DK. Choroid plexus papillomas of the cerebellopontine angle. Neurol India. 2002. 50: 352-8
18. Maria BL, Graham ML, Strauss LC, Wharam MD. Response of a recurrent choroid plexus tumor to combination chemotherapy. J Neurooncol. 1985. 3: 259-62
19. McGirr SJ, Ebersold MJ, Scheithauer BW, Quast LM, Shaw EG. Choroid plexus papillomas: Long-term follow-up results in a surgically treated series. J Neurosurg. 1988. 69: 843-9
20. Menon G, Nair SN, Baldawa SS, Rao RB, Krishnakumar KP, Gopalakrishnan CV. Choroid plexus tumors: An institutional series of 25 patients. Neurol India. 2010. 58: 429-35
21. Mishra A, Ojha BK, Chandra A, Singh SK, Chandra N, Srivastava C. Choroid plexus papilloma of posterior third ventricle: A case report and review of literature. Asian J Neurosurg. 2014. 9: 238-
22. Nakano I, Kondo A, Iwasaki K. Choroid plexus papilloma in the posterior third ventricle: Case report. Neurosurgery. 1997. 40: 1279-82
23. Ogiwara H, Dipatri AJ, Alden TD, Bowman RM, Tomita T. Choroid plexus tumors in pediatric patients. Br J Neurosurg. 2012. 26: 32-7
24. Pascual-Castroviejo I, Roche MC, Villarejo F, Gutierrez Molina M, Pérez Higueras A. Choroid plexus papillomas of the fourth ventricle. Report of 3 cases. Childs Brain. 1982. 9: 373-80
25. Pawar SJ, Sharma RR, Mahapatra AK, Lad SD, Musa MM. Choroid plexus papilloma of the posterior third ventricle during infancy and childhood: Report of two cases with management morbidities. Neurol India. 2003. 51: 379-82
26. Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Brunelle F, Sgouros S. Papillomas and carcinomas of the choroid plexus in children. J Neurosurg. 1998. 88: 521-8
27. Phi JH, Goobie SM, Hong KH, Dholakia A, Smith ER. Use of tranexamic acid in infants undergoing choroid plexus papilloma surgery: A report of two cases. Paediatr Anaesth. 2014. 24: 791-3
28. Piguet V, de Tribolet N. Choroid plexus papilloma of the cerebellopontine angle presenting as a subarachnoid hemorrhage: Case report. Neurosurgery. 1984. 15: 114-6
29. Pratheesh R, Moorthy RK, Singh R, Rajshekhar V. Choroid plexus papilloma presenting as a non-contrast-enhancing fourth ventricular mass in a child. Neurol India. 2009. 57: 486-8
30. Raimondi AJ, Gutierrez FA. Diagnosis and surgical treatment of choroid plexus papillomas. Childs Brain. 1975. 1: 81-115
31. Rosai J.editorsRosai and Ackerman's Surgical Pathology. New York: Mosby; 2004. p.
32. Rostasy KM, Sponholz S, Bahn E, Ludwig HC, Hanefeld F. Unusual localization of a choroid plexus papilloma in a 4-year-old female. Pediatr Neurol. 2003. 28: 66-8
33. Rovit RL, Schechter MM, Chodroff P. Choroid plexus paillomas. Observations on radiographic diagnosis. Am J Roentgenol Radium Ther Nucl Med. 1970. 110: 608-17
34. Safaee M, Clark AJ, Bloch O, Oh MC, Singh A, Auguste KI. Surgical outcomes in choroid plexus papillomas: An institutional experience. J Neurooncol. 2013. 113: 117-25
35. Sarkar C, Sharma MC, Gaikwad S, Sharma C, Singh VP. Choroid plexus papilloma: A clinicopathological study of 23 cases. Surg Neurol. 1999. 52: 37-9
36. Sasani M, Solmaz B, Oktenoglu T, Ozer AF. An unusual location for a choroid plexus papilloma: The pineal region. Childs Nerv Syst. 2014. 30: 1307-11
37. Shin JH, Lee HK, Jeong AK, Park SH, Choi CG, Suh DC. Choroid plexus papilloma in the posterior cranial fossa: MR, CT, and angiographic findings. Clin Imaging. 2001. 25: 154-62
38. Shuto T, Sekido K, Ohtsubo Y, Tanaka Y, Hara M, Yamaguchi K. Choroid plexus papilloma of the III ventricle in an infant. Childs Nerv Syst. 1995. 11: 664-6
39. Spallone A, Pastore FS, Hagi Mao M. Choroid plexus papillomas of the cerebellopontine angle in a child. Ital J Neurol Sci. 1986. 7: 613-6
40. Tacconi L, Delfini R, Cantore G. Choroid plexus papillomas: Consideration of a surgical series of 33 cases. Acta Neurochir (Wien). 1996. 138: 802-10
41. Talacchi A, De Micheli E, Lombardo C, Turazzi S, Bricolo A. Choroid plexus papilloma of the cerebellopontine angle: A twelve patient series. Surg Neurol. 1999. 51: 621-9
42. Trivelato FP, Manzato LB, Rezende MT, Barroso PM, Faleiro RM, Ulhôa AC. Preoperative embolization of choroid plexus papilloma with Onyx via the anterior choroidal artery: Technical note. Childs Nerv Syst. 2012. 28: 1955-8
43. Tsumoto T, Nakai E, Uematsu Y, Nakai K, Itakura T. Choroid plexus papilloma in the posterior third ventricle in infancy: A case report. No Shinkei Geka. 1999. 27: 673-8
44. Vazquez E, Ball WS, Prenger EC, Castellote A, Crone KR. Magnetic resonance imaging of fourth ventricular choroid plexus neoplasms in childhood. A report of two cases. Pediatr Neurosurg. 1991-1992. 17: 48-52