- Department of Neurosurgery, Prasat Neurological Institute, Bangkok, Thailand.
- Department of Neuroradiology, Prasat Neurological Institute, Bangkok, Thailand.
- Department of Radiology, Bumrungrad International Hospital, Bangkok, Thailand.
Correspondence Address:
Prasert Iampreechakul, Department of Neurosurgery, Prasat Neurological Institute Bangkok, Thailand.
DOI:10.25259/SNI_612_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prasert Iampreechakul1, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Cauda equina arteriovenous fistula supplied by proximal radicular artery and concomitant sacral dural arteriovenous fistula: A case report and literature review. 16-Aug-2021;12:405
How to cite this URL: Prasert Iampreechakul1, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Cauda equina arteriovenous fistula supplied by proximal radicular artery and concomitant sacral dural arteriovenous fistula: A case report and literature review. 16-Aug-2021;12:405. Available from: https://surgicalneurologyint.com/surgicalint-articles/11051/
Abstract
Background: Cauda equina arteriovenous fistulas (AVFs) fed by the proximal radicular artery are exceedingly rare. Spinal dural arteriovenous fistulas (DAVFs) in the sacral region are rare and usually misdiagnosed. We report a case of a cauda equina AVF with concomitant sacral DAVF. We also review the coexistence of multiple types of spinal vascular malformations in a single patient.
Case Description: A 54-year-old man presented with progressive weakness of the lower extremities for 1 month. Magnetic resonance imaging (MRI) of the lumbosacral and thoracic spine showed spinal cord congestion, extending from the conus medullaris to the level of T7, and abnormal tortuous and dilated flow void, running from the level of L5 to T12 along anterior surface of the spinal cord. Spinal angiography demonstrated the fistula at the level of L2 below the conus medullaris. Based on intraoperative findings, the cauda equina AVF supplied by the proximal radicular artery with cranial drainage through the enlarged radicular vein was confirmed and successfully obliterated. Another enlarged arterialized radicular vein running parallel to another cauda equina nerve root is observed with unknown origin. After the operation, the patient showed mild improvement of his symptoms. Follow-up MRI and contrast-enhanced MR angiography revealed an another sacral DAVF vascularized by the lateral sacral artery.
Conclusion: The coexistence of different spinal vascular malformations in a same patient is extremely rare. Most authors of several studies hypothesized that venous hypertension and thrombosis due to the presence or treatment of the first spinal vascular lesion may produce a second DAVF.
Keywords: Cauda equina arteriovenous fistula, Filum terminale arteriovenous fistula, Multiple spinal vascular malformations, Radicular arteriovenous fistula, Sacral dural arteriovenous fistula
INTRODUCTION
Spinal vascular malformations have been classified into four subtypes including Type I, spinal dural arteriovenous fistulas (SDAVFs); Type II, intramedullary glomus malformations; Type III, extensive juvenile malformations; and Type IV, intradural perimedullary arteriovenous fistulas (PMAVFs).[
Cauda equina arteriovenous fistulas (AVFs) or radicular AVFs (rAVFs) are fistulas located on a nerve root of the cauda equina intradurally and quite rare.[
SDAVFs are the most common type of spinal vascular malformations.[
The coexisting of the same type of spinal vascular malformations has been reported.[
The authors described a patient with concomitant cauda equina AVF fed by proximal radicular artery and sacral DAVF. The authors also reviewed the published case reports and series that enough clinical description and clearly demonstrated figures of the coexistence of multiple types of spinal vascular lesions. This literature search was performed on the Ovid MEDLINE, PubMed, Cochrane Database of Systemic Reviews, and Google Scholar with the following key words of “concomitant,” “multiple,” “coexistence,” “concurrent,” “spinal,” “vascular malformations,” and “AVF.” Exclusion criteria included non-English-language articles, commentaries, and information from expert opinions.
CASE DESCRIPTION
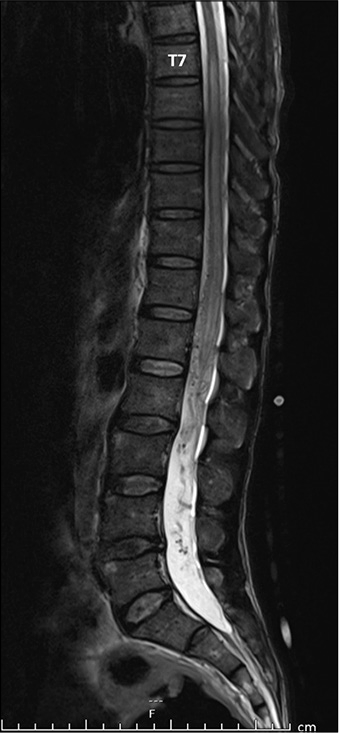
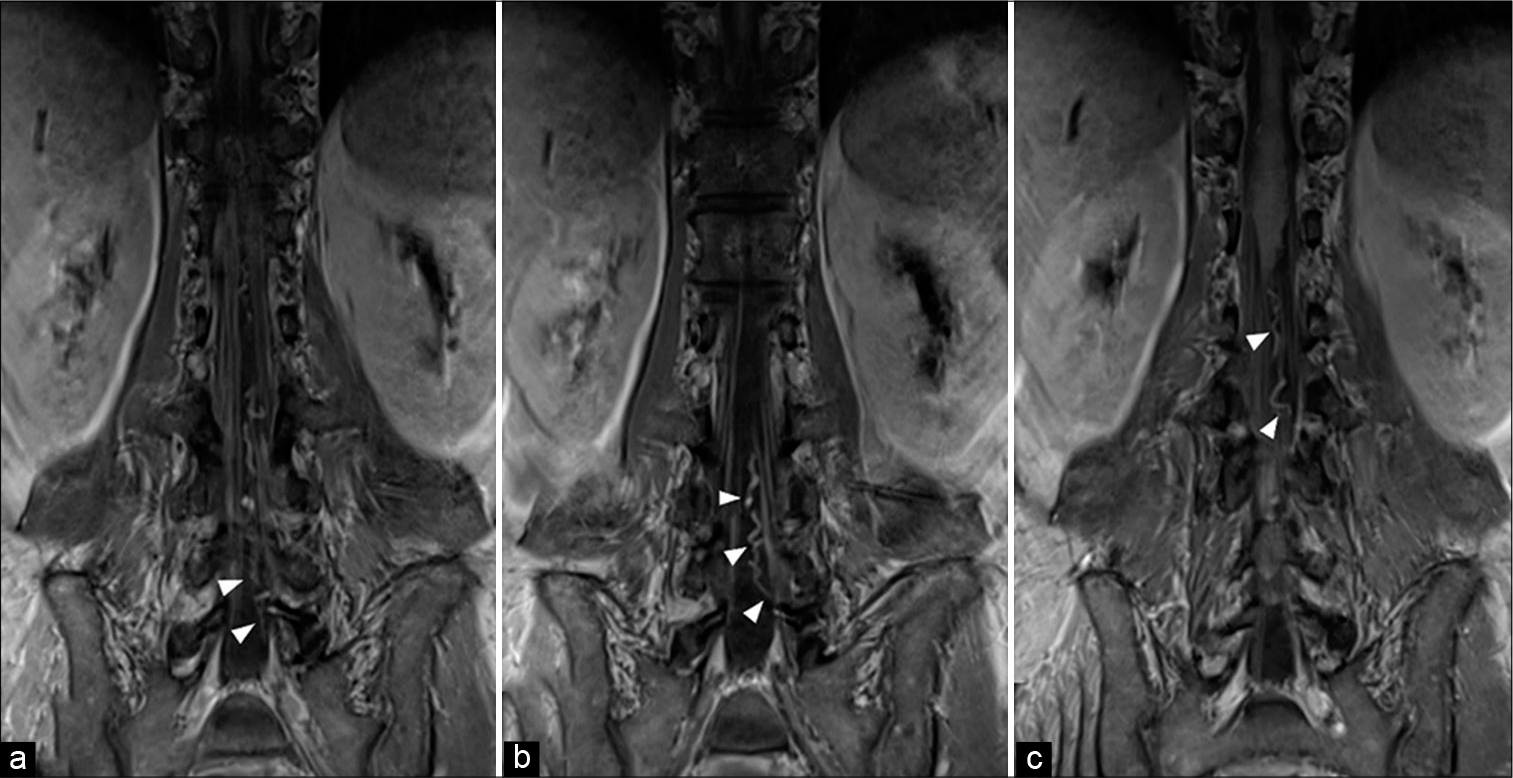
A 54-year-old man, heavy smoker without underlying disease, was admitted to the local hospital due to progressive weakness of the lower extremities for 1 month. He had no history any injury. Three months earlier, he experienced low back pain radiating to both legs, predominantly affecting the left side. Furthermore, difficulty in urination and constipation were observed 1 week before admission. Magnetic resonance imaging (MRI) of the lumbosacral and thoracic spine revealed abnormal hyperintense T2 signal, representing spinal cord congestion, extending from the conus medullaris to the level of T7. There was abnormal tortuous and dilated flow void, running from the level of L5 to T12 along anterior surface of the spinal cord [
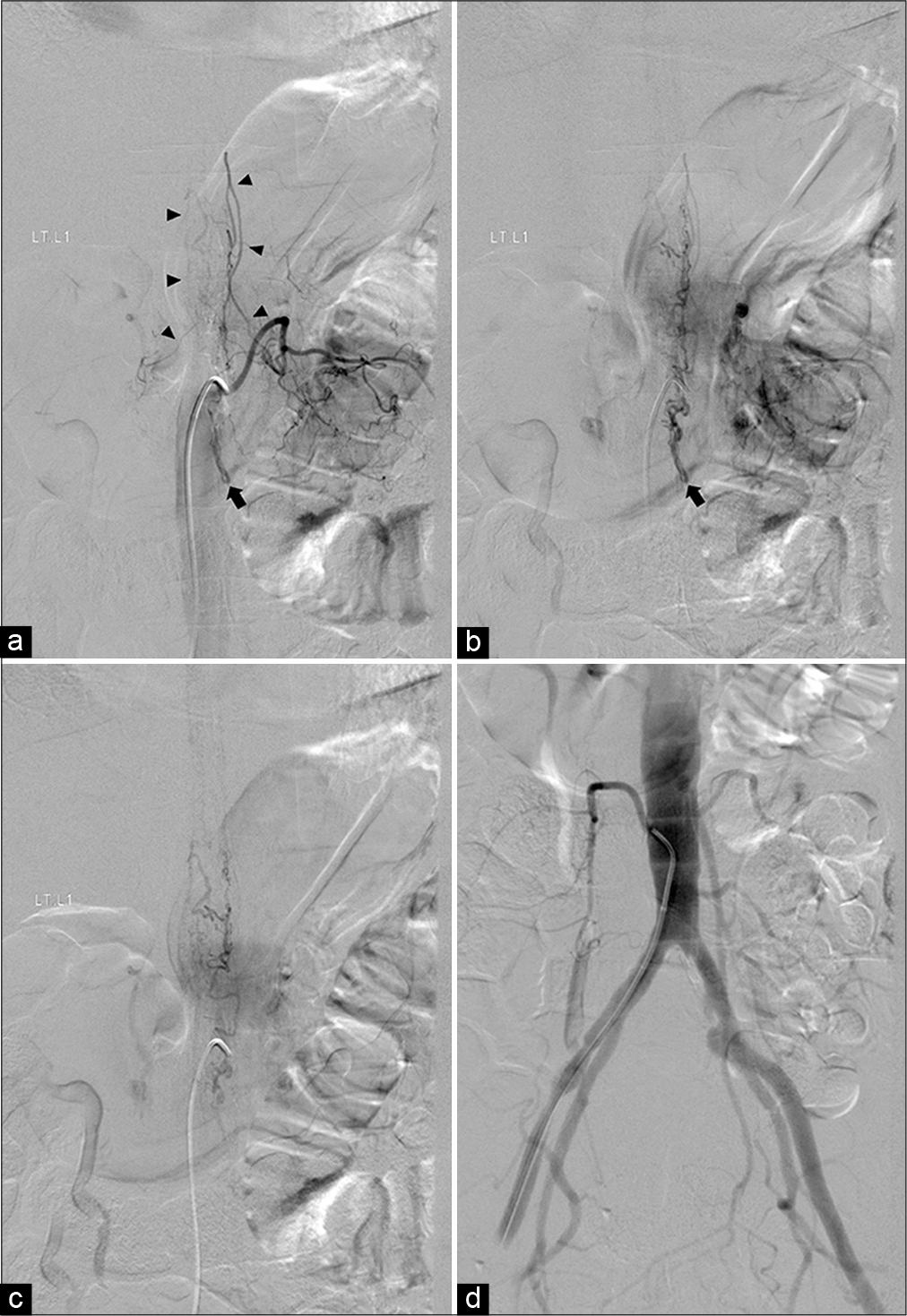
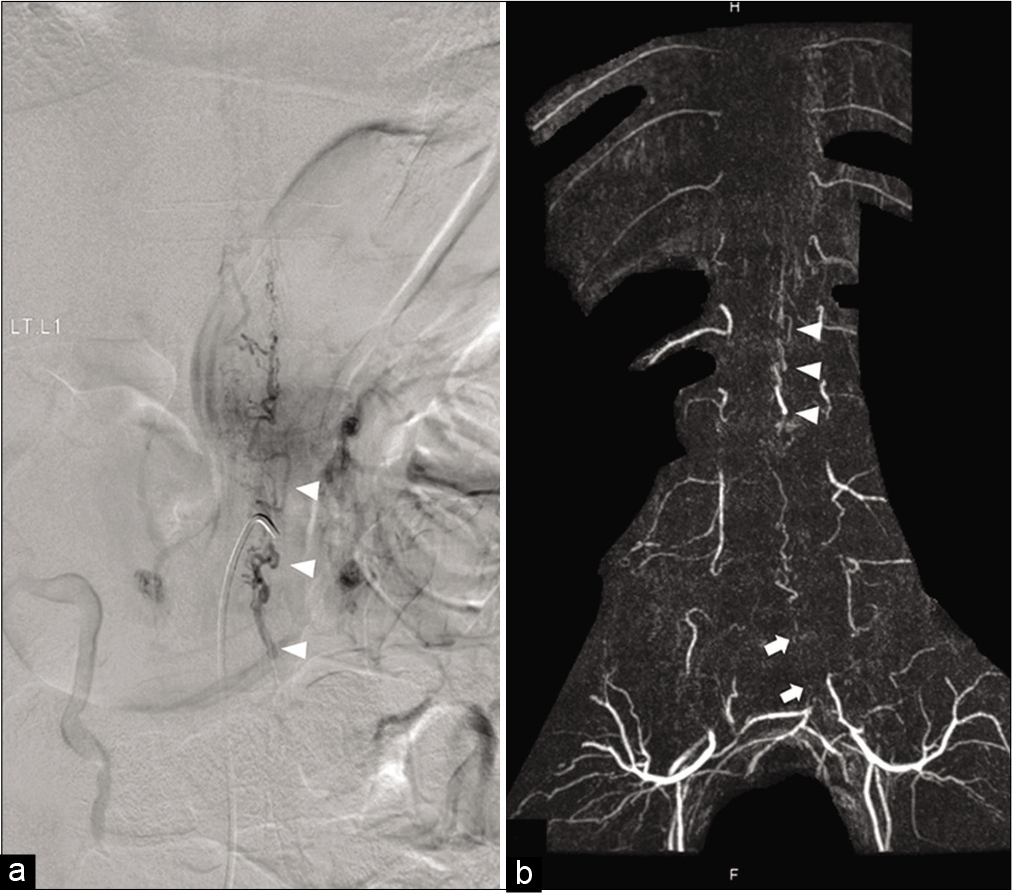
Spinal angiography demonstrated the fistula at the level of L2 below the conus medullaris, which is supplied by the PSA originating from the left L1 segmental artery with cranial drainage through the paralleling dilated vein into perimedullary vein. Without selective catheterization into both internal iliac arteries, lower aorta and bilateral common iliac arteries angiography reveals no more supply to the fistula [
Figure 2:
Anteroposterior views of the left L1 segmental artery angiography in (a) early, (b) middle, and (c) late phases demonstrate filling of the posterior spinal artery supplying the fistula (arrow), located at the level of L2 below the arterial basket of the conus medullaris forming from the bilateral posterior spinal arteries (arrowheads), with cranial drainage into perimedullary veins. (d) Anteroposterior view of flush aortography including lower aorta and bilateral iliac arteries reveals no more supply to the fistula.
Figure 3:
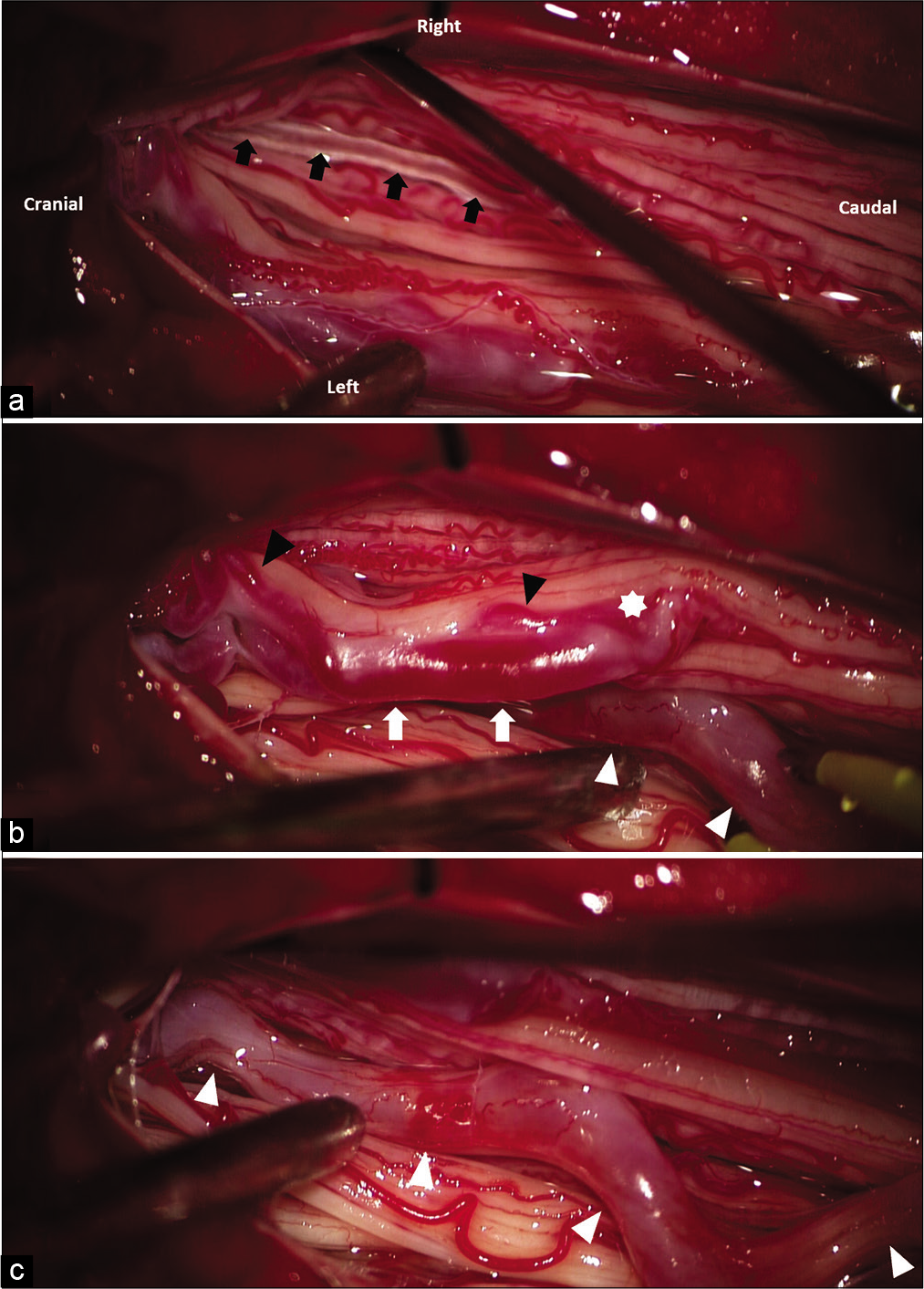
Intraoperative photograph during surgery on prone position after opening the dura. (a) Normal vessels on the filum terminale (black arrows). (b) The fistula is located on the left cauda equina nerve root (asterisk) supplied by the feeding artery (black arrowhead) with cranial drainage through the dilated radicular vein (white arrow) (c) another enlarged arterialized radicular vein (white arrowhead) running parallel to the cauda equina nerve root is observed with unknown origin.
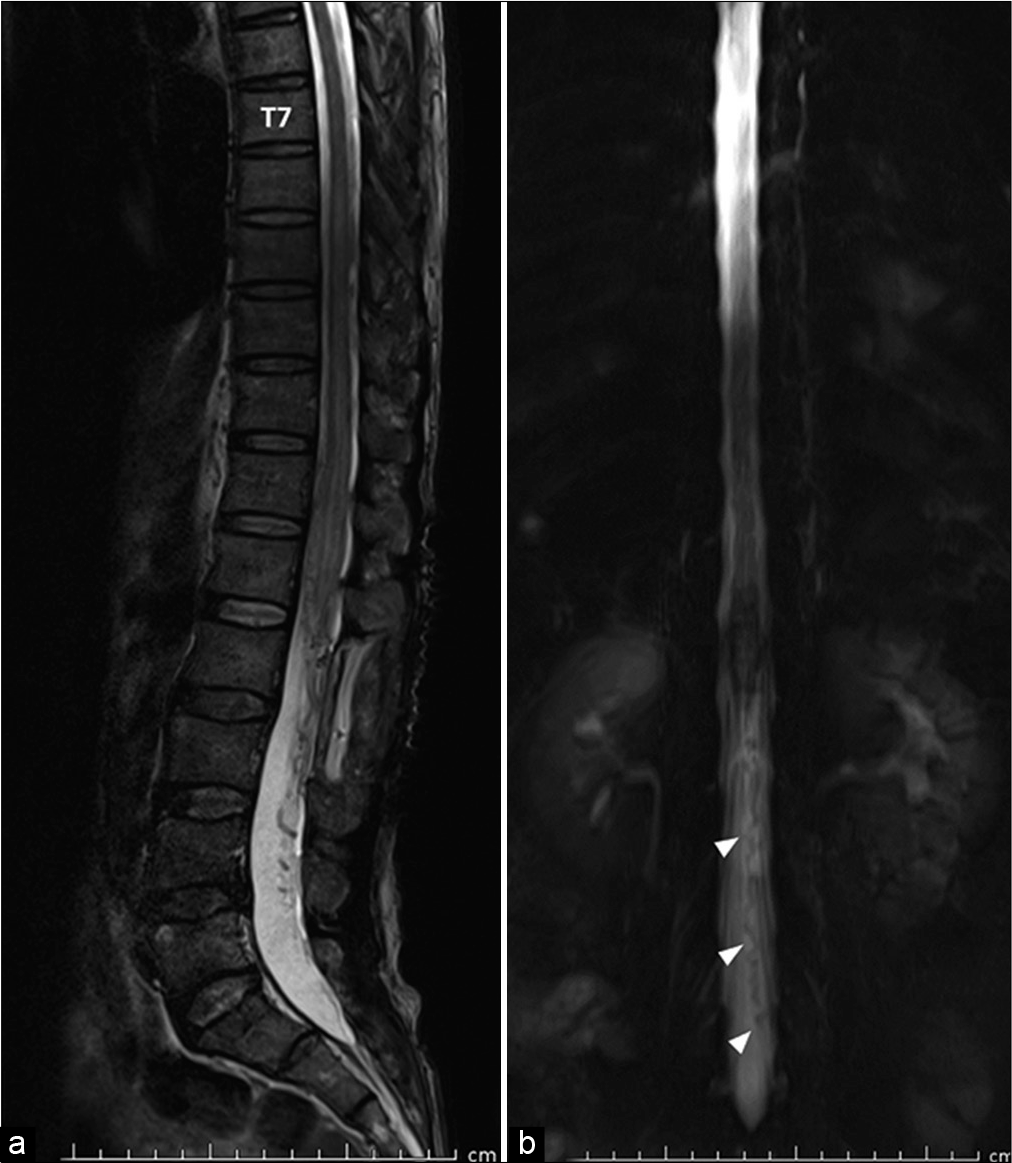
Follow-up MRI and contrast-enhanced MR angiography (MRA) of the thoracolumbar spine, obtained 3 weeks after the operation, revealed mild regression of spinal cord congestion, and remaining of intradural flow void from L5 to L2. Another SDAVF was found at left S1 neural foramen supplied by the left lateral sacral artery (LSA) originating from the left internal iliac artery with venous drainage into perimedullary veins through the dilated and tortuous radicular vein, probably corresponding with another dilated arterialized radicular vein found during the operation [
Figure 4:
(a) Sagittal T2-weighted image of the thoracolumbar spine obtained 3 weeks after the operation reveals mild regression of spinal cord congestion and remaining of intradural flow void from L5 to L2. (b) Coronal magnetic resonance myelography of the lumbosacral spine demonstrates abnormal flow void running from the left sacral nerve root to the left-sided conus medullaris, probably representing a dilated radicular vein.
Figure 6:
Comparing between anteroposterior view of (a) preoperative the left L1 segmental artery angiography in late venous phase and (b) postoperative contrast-enhanced magnetic resonance angiography demonstrates the same venous drainage pattern (arrowheads), representing sharing the common medullary venous channel. The feeder from the left lateral sacral artery (arrows) is noted.
DISCUSSION
The cauda equina AVF
Based on the study of vascular anatomy of the cauda equina by Namba,[
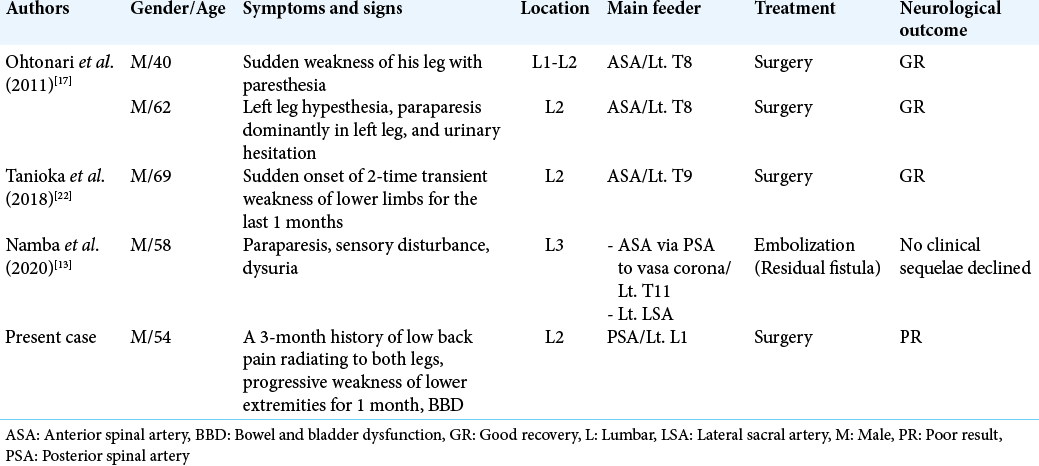
Cauda equina AVFs supplied by proximal radicular arteries are extremely rare. After review of the literature, there were only five cases, including our one case [
Hong et al.[
To avoid treatment-related injury to the cauda equina during endovascular treatment, surgery is the best choice for the fistula of the cauda equina nerve root.[
The concomitant different types of spinal vascular malformations
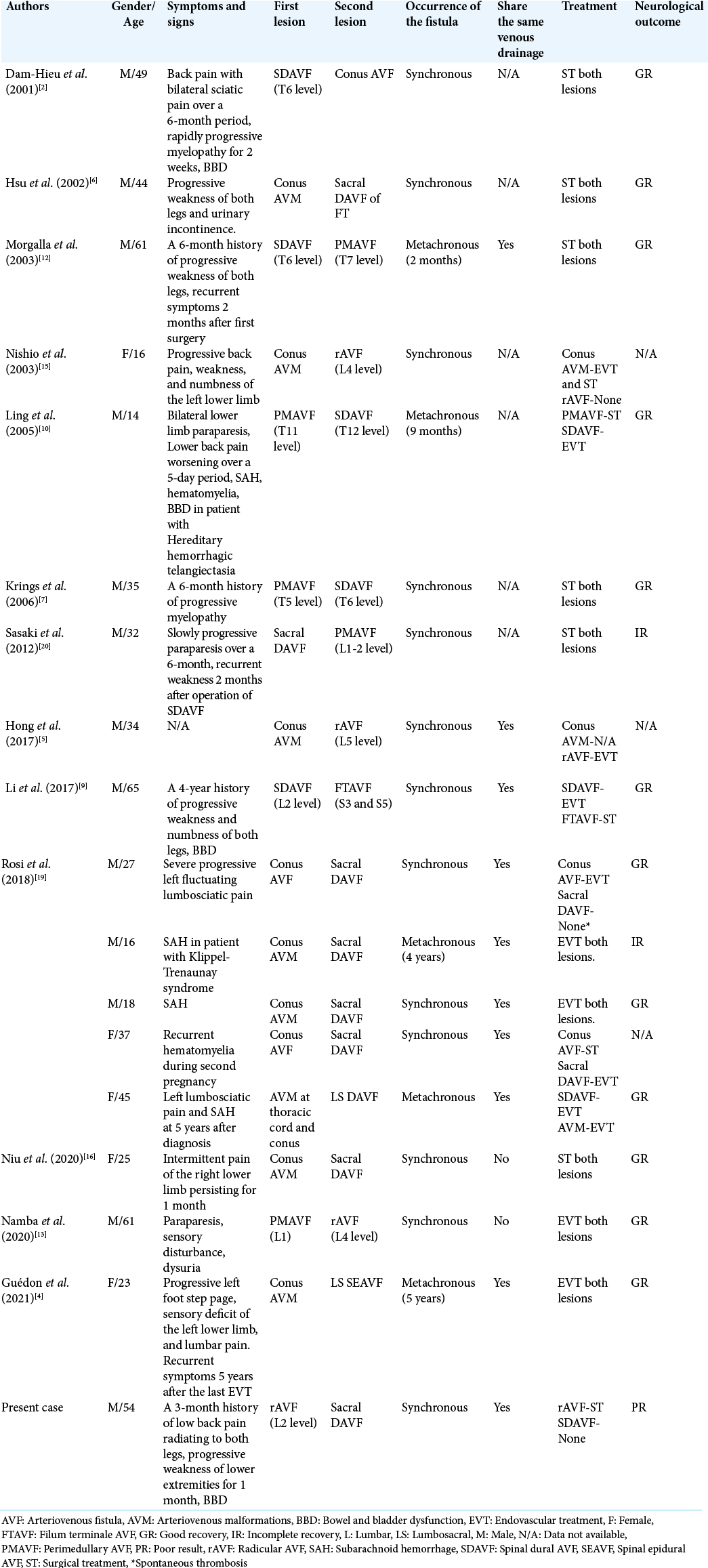
Owing to the rarity of concurrent different spinal vascular malformations in a single patient, the diagnosis of two lesions at the same time remains challenging, as shown in our case. According to our review about the coexistence of multiple types of spinal vascular lesions, the collected data in this review included demographic data (i.e., patient gender and age), symptoms and signs, type with location of each fistula, synchronous or metachronous occurrence of these fistulas, the presence of sharing the same venous drainage, treatment, and neurological outcome of the patients [
The pathogenesis of the concurrent different spinal vascular malformations in different spinal levels
The relationship between the two different spinal vascular malformations remains unknown. Whether the concurrence is purely a coincidence or pathogenetic linked is unclear.[
In 2001, Dam-Hieu et al.[
In the present study, the cauda equina AVF and sacral DAVF linked a common medullary venous channel. We speculated that primary rAVF may promote medullary venous congestion and subsequent stagnation or thrombosis inducting of a secondary sacral DAVF.
Management of the concurrent different spinal vascular malformations
The treatment of multifocal different spinal vascular malformations coexisting in the same patient remains unclear and usually difficult, especially in two lesions with distance locations. The lesion responsible for the clinical symptoms needs to be identified and treated first. However, in case of both lesions were possibly responsible for the clinical symptoms; the treatment of both lesions in the same session may be the best choice.[
Rarely, spontaneous regression of sacral DAVF occurred following embolization of conus AVF due to the thrombosis of the venous system shared by both pial and dural lesions. However, this strategy may be ineffective, and the patient may develop worsening symptoms from the other lesions left untreated.[
Pitfalls of diagnostic studies
It is commonly accepted that spinal angiography is complete when one fistula has been discovered and the ASA has been visualized. However, this concept may not be sufficient in all patients. The extent and direction of the venous drainage visualized by MRI and spinal angiography should be evaluated for discrepancies.[
In the present study, we found three pitfalls of diagnostic studies. First, we missed a concurrent sacral DAVF on initial spinal angiography after discovering the first fistula. Secondly, the presence of intradural hypointense vessel or flow voids in lumbar region or below the conus medullaris on T2-weghted MRI should be considered as a clue for awareness of a possible sacral DAVF. Even though we found one fistula at the level of L2, but we could not identify the venous drainage below the conus medullaris seen on MRI. Therefore, we should keep in mind to find another possible fistula even extremely rare condition. Another last pitfall, selective catheterization of the internal iliac arteries is imperative for confirming the fistula in the sacral region supplied by the LSA. The fistula usually cannot be identified with flush aortography or selective injection of the common iliac arteries.[
Some authors suggested that complete spinal angiography of all intersegmental arteries from the craniocervical junction to sacrum should be performed to clear of concurrent AVF.[
After treatment one spinal vascular lesion, if the clinical status of patient fails to improve or deteriorates further with persistent flow voids seen on postoperative MR images, the possibility of reopening of the lesion or the presence of an associated malformations at different spinal level should be considered and repeat complete angiography must be performed, especially if the initial angiography was incomplete.[
CONCLUSION
The presence of different spinal vascular malformations in a same patient is extremely rare. However, the clinician should be aware of the possibility of this condition. From our review, most authors of several studies hypothesized that venous hypertension and thrombosis due to the presence or treatment of the first spinal vascular lesion may produce a second DAVF.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anson JA, Spetzler RF. Classification of spinal arteriovenous malformations and implications for treatment. BNI Q. 1992. 8: 2-8
2. Dam-Hieu P, Mineo JF, Bostan A, Nonent M, Besson G. Concurrent spinal dural and intradural arteriovenous fistulas. Case report. J Neurosurg. 2001. 95: 96-9
3. Gioppo A, Faragò G, Giannitto C, Caputi L, Saladino A, Acerbi F. Sacral dural arteriovenous fistulas: A diagnostic and therapeutic challenge-single-centre experience of 13 cases and review of the literature. J Neurointerv Surg. 2018. 10: 415-21
4. Guédon A, Condette-Auliac S, Consoli A, di Maria F, Coskun O, Rodesch G. Primary conus medullaris arteriovenous shunt and secondary lumbo-sacral epidural arteriovenous fistula: One malformation can hide another. J Neuroradiol. 2021. 48: 16-20
5. Hong T, Park JE, Ling F, terBrugge KG, Tymianski M, Zhang HQ. Comparison of 3 different types of spinal arteriovenous shunts below the conus in clinical presentation, radiologic findings, and outcomes. AJNR Am J Neuroradiol. 2017. 38: 403-9
6. Hsu SW, Rodesch G, Luo CB, Chen YL, Alvarez H, Lasjaunias PL. Concomitant conus medullaris arteriovenous malformation and sacral dural arteriovenous fistula of the filum terminale. Interv Neuroradiol. 2002. 8: 47-53
7. Krings T, Coenen VA, Weinzierl M, Reinges MH, Mull M, Thron A. Spinal dural arteriovenous fistula associated with a spinal perimedullary fistula: Case report. J Neurosurg Spine. 2006. 4: 241-5
8. Larsen DW, Halbach VV, Teitelbaum GP, McDougall CG, Higashida RT, Dowd CF. Spinal dural arteriovenous fistulas supplied by branches of the internal iliac arteries. Surg Neurol. 1995. 43: 35-40
9. Li J, Li G, Bian L, Hong T, Yu J, Zhang H. Concomitant lumbosacral perimedullary arteriovenous fistula and spinal dural arteriovenous fistula. World Neurosurg. 2017. 105: 1041.e7-14
10. Ling JC, Agid R, Nakano S, Souza MP, Reintamm G, Terbrugge KG. Metachronous multiplicity of spinal cord arteriovenous fistula and spinal dural AVF in a patient with hereditary haemorrhagic telangiectasia. Interv Neuroradiol. 2005. 11: 79-82
11. Matsumaru Y, Pongpech S, Laothamas J, Alvarez H, Rodesch G, Lasjaunias P. Multifocal and metameric spinal cord arteriovenous malformations. Review of 19 cases Interv Neuroradiol. 1999. 5: 27-34
12. Morgalla MH, Ernemann U, Gawlowski J, Deininger M, Bitzer M, Grote EH. Recurrent spinal fistula as result of a rare combination of a perimedullary and peridural spinal fistula: Case report. Surg Neurol. 2004. 61: 347-52
13. Namba K, Niimi Y, Ishiguro T, Higaki A, Toma N, Komiyama M. Cauda equina and filum terminale arteriovenous fistulas: Anatomic and radiographic features. AJNR Am J Neuroradiol. 2020. 41: 2166-70
14. Namba K. Vascular anatomy of the cauda equina and its implication on the vascular lesions in the caudal spinal structure. Neurol Med Chir (Tokyo). 2016. 56: 310-6
15. Nishio A, Ohata K, Takami T, Gotoh T, Tsuyuguchi N, Ichinose T. Spinal arteriovenous malformation associated with a radicular arteriovenous fistula suggested a metameric disease. A case report. Interv Neuroradiol. 2003. 9: 75-8
16. Niu X, Ren Y, Li J. Concomitant sacral dural arteriovenous fistula and conus medullaris arteriovenous malformation with respective drainage veins: Case report and literature review. World Neurosurg. 2020. 141: 299-305
17. Ohtonari T, Ota S, Nishihara N, Suwa K, Ota T, Sekihara Y. Arteriovenous fistula in a nerve root of the cauda equina fed by a proximal radiculo-medullary artery: A report of two cases. Interv Neuroradiol. 2011. 17: 217-23
18. Pierot L, Vlachopoulos T, Attal N, Martin N, Bert S, Chiras J. Double spinal dural arteriovenous fistulas: Report of two cases. AJNR Am J Neuroradiol. 1993. 14: 1109-12
19. Rosi A, Consoli A, Condette-Auliac S, Coskun O, di Maria F, Rodesch G. Concomitant conus medullaris arteriovenous shunts and sacral dural arteriovenous fistulas: Pathophysiological links related to the venous drainage of the lesions in a series of five cases. J Neurointerv Surg. 2018. 10: 586-92
20. Sasaki O, Yajima N, Ichikawa A, Yamashita S, Nakamura K. Deterioration after surgical treatment of spinal dural arteriovenous fistula associated with spinal perimedullary fistula. Neurol Med Chir (Tokyo). 2012. 52: 516-20
21. Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002. 96: 145-56
22. Tanioka S, Toma N, Sakaida H, Umeda Y, Suzuki H. A case of arteriovenous fistula of the cauda equina fed by the proximal radicular artery: Anatomical features and treatment precautions. Eur Spine J. 2018. 27: 281-6