- Department of Neurosurgery, Graduate School of Medicine, Suita, Osaka, Japan,
- Department of Gastroenterology and Hepatology, Graduate School of Medicine, Suita, Osaka, Japan,
- Department of Pathology, Osaka University, Graduate School of Medicine, Suita, Osaka, Japan.
Correspondence Address:
Noriyuki Kijima, Department of Neurosurgery, Osaka University, Graduate School of Medicine, Suita, Osaka, Japan.
DOI:10.25259/SNI_117_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shuhei Yamada1, Noriyuki Kijima1, Manabu Kinoshita1, Shinichiro Shinzaki2, Kazuaki Sato3, Kansuke Kido3, Ryuichi Hirayama1, Naoki Kagawa1, Tetsuo Takehara2, Eiichi Morii3, Haruhiko Kishima1. Cerebellopontine angle metastasis of a neuroendocrine tumor mimicking vestibular schwannoma: A case report. 23-Jun-2022;13:264
How to cite this URL: Shuhei Yamada1, Noriyuki Kijima1, Manabu Kinoshita1, Shinichiro Shinzaki2, Kazuaki Sato3, Kansuke Kido3, Ryuichi Hirayama1, Naoki Kagawa1, Tetsuo Takehara2, Eiichi Morii3, Haruhiko Kishima1. Cerebellopontine angle metastasis of a neuroendocrine tumor mimicking vestibular schwannoma: A case report. 23-Jun-2022;13:264. Available from: https://surgicalneurologyint.com/surgicalint-articles/11671/
Abstract
Background: Neuroendocrine tumors (NETs) are uncommon neoplasms arising from neuroendocrine cells and are rarely associated with intracranial metastases.
Case Description: We discuss the case of a 74-year-old woman with a right CPA tumor. She had a history of retroperitoneal NET, but was diagnosed with vestibular schwannoma due to a right-sided hearing loss and a right CPA tumor along the VII and VIII nerves. After a 3-year follow-up, she presented with repetitive vomiting, a 1-month history of gait instability, and a 3-month history of general fatigue. Brain imaging revealed tumor growth and edematous changes in the right cerebellum. She underwent retrosigmoid craniotomy and partial resection. Histopathological examination revealed metastatic NET. She underwent stereotactic radiosurgery for residual lesion and, at 11 months of follow-up, the lesion was confirmed to have shrunk on magnetic resonance imaging (MRI).
Conclusion: This is the first case to report the natural course of cerebellopontine metastasis of a NET. The differential diagnosis of CPA tumors is diverse, and, in our case, we suspected a vestibular schwannoma because of the typical symptoms and imaging features. However, the tumor grew relatively faster than expected and showed intratumoral hemorrhage during the 3-year follow-up. Therefore, in patients with a history of a NET, a careful follow-up is advisable even for lesions highly suspected to be another benign tumor on MRI. Careful follow-up imaging and appropriate treatment strategies were useful to manage the brain metastasis. Although NETs metastasizing to the CPA are extremely rare, this possibility should be considered when patients with NETs have intracranial lesions.
Keywords: Brain metastasis, Case report, Cerebellopontine angle tumor, Neuroendocrine tumor, Neuroendocrine tumors, Vestibular schwannoma
INTRODUCTION
Neuroendocrine tumors (NETs) are uncommon neoplasms arising from neuroendocrine cells. The overall incidence is an estimated 1–4 patients/100,000 population/year.[
CASE DESCRIPTION
A 74-year-old woman presented to our department for the follow-up of a brain metastasis from a NET. At 65 years of age, she had abdominal pain and gross hematuria, was diagnosed with a primary retroperitoneal NET, and underwent laparotomy at another hospital. She had a left obturator lymph node metastasis at the time of diagnosis and had lung metastasis at 69 years of age. She had been administered octreotide at 70 years, carboplatin and etoposide at 72, and streptozotocin at 73, but all these drugs were discontinued due to disease progression. At 74, she was diagnosed with brain metastases and underwent tumor resection in the left parietal and right frontal lobes at another hospital.
When she presented to our department, a right CPA tumor was observed, and she had a right-sided hearing loss. Since it was located along the VII and VIII nerves and the right internal auditory canal appeared dilated on imaging, it was diagnosed as vestibular schwannoma [
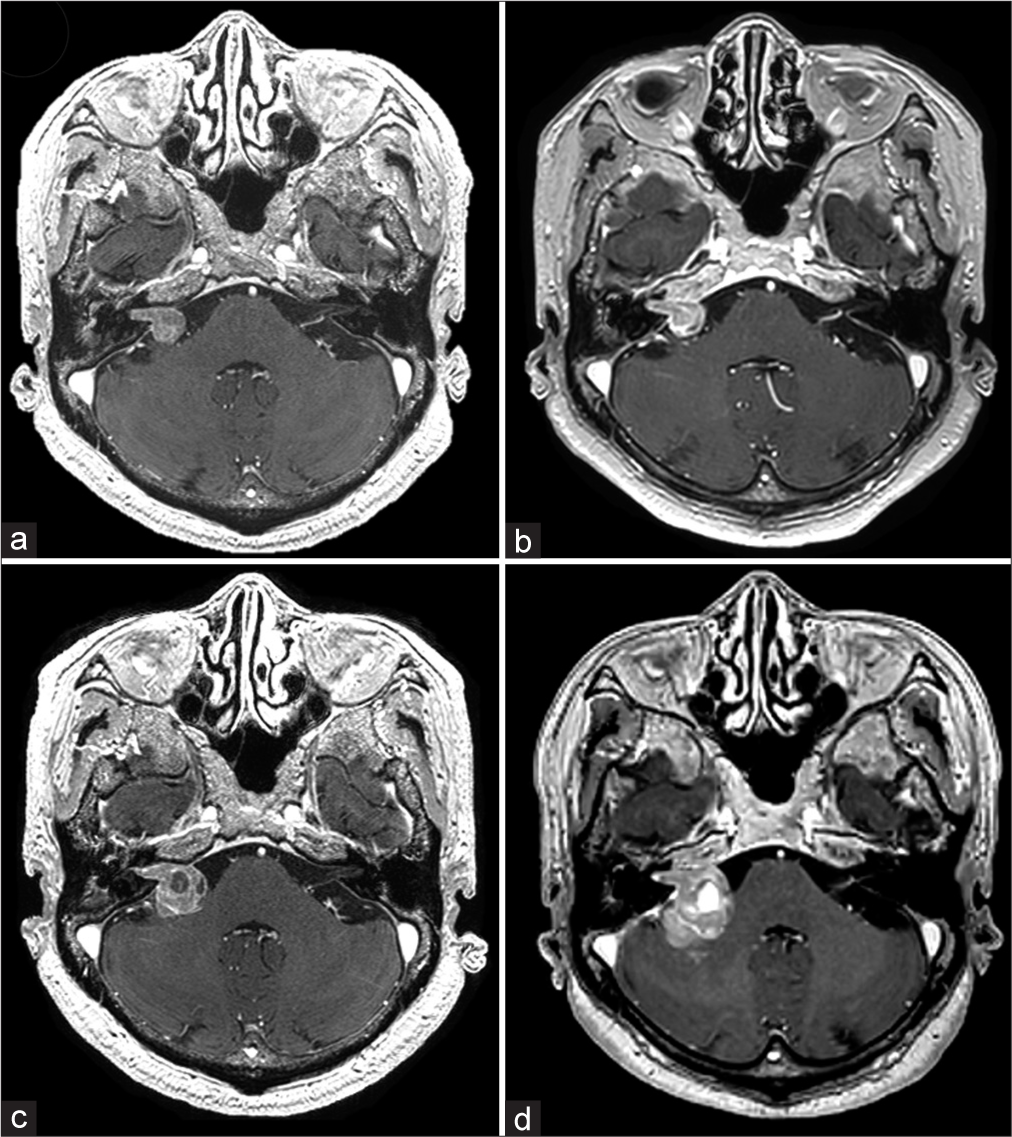
Figure 1:
Contrast-enhanced magnetic resonance imaging (CE-MRI) findings of the cerebellopontine angle lesion. (a) Images taken on the first consultation showed a suspicious lesion of about 12 mm in size, resembling a vestibular schwannoma, at the right cerebellopontine angle. (b) CE-MRI images 2 years after the first consultation (13 months before admission) showed no change in the tumor size. (c) CE-MRI images taken 7 months before admission showed an intratumoral cyst and tumor growth to 20 mm. (d) CE-MRI taken 1 month before admission showed intratumoral hemorrhage, edematous changes in the right cerebellum, and tumor growth to 27 mm.
Computed tomography (CT) scan and MRI revealed tumor growth, edematous changes in the right cerebellum, and hydrocephalus [
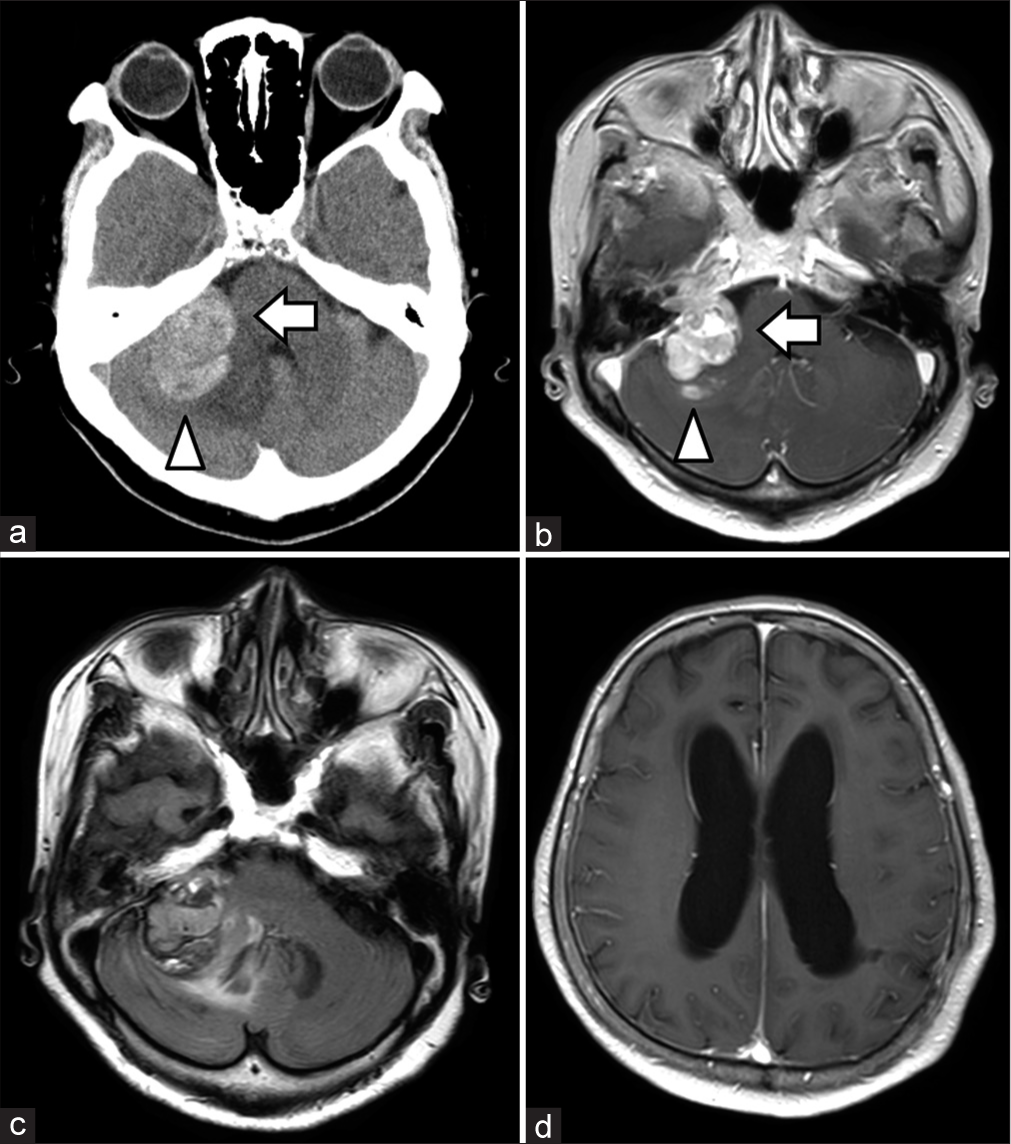
Figure 2:
Imaging findings on the day of admission. Computed tomography scan (a), contrast-enhanced magnetic resonance imaging (CE-MRI) (b), and fluid-attenuated inversion recovery (c) images on the day of admission showing the tumor (arrow), hemorrhage (arrowhead), and extensive edematous changes in the right cerebellum. CE-MRI (d) showed the enlargement of lateral ventricles.
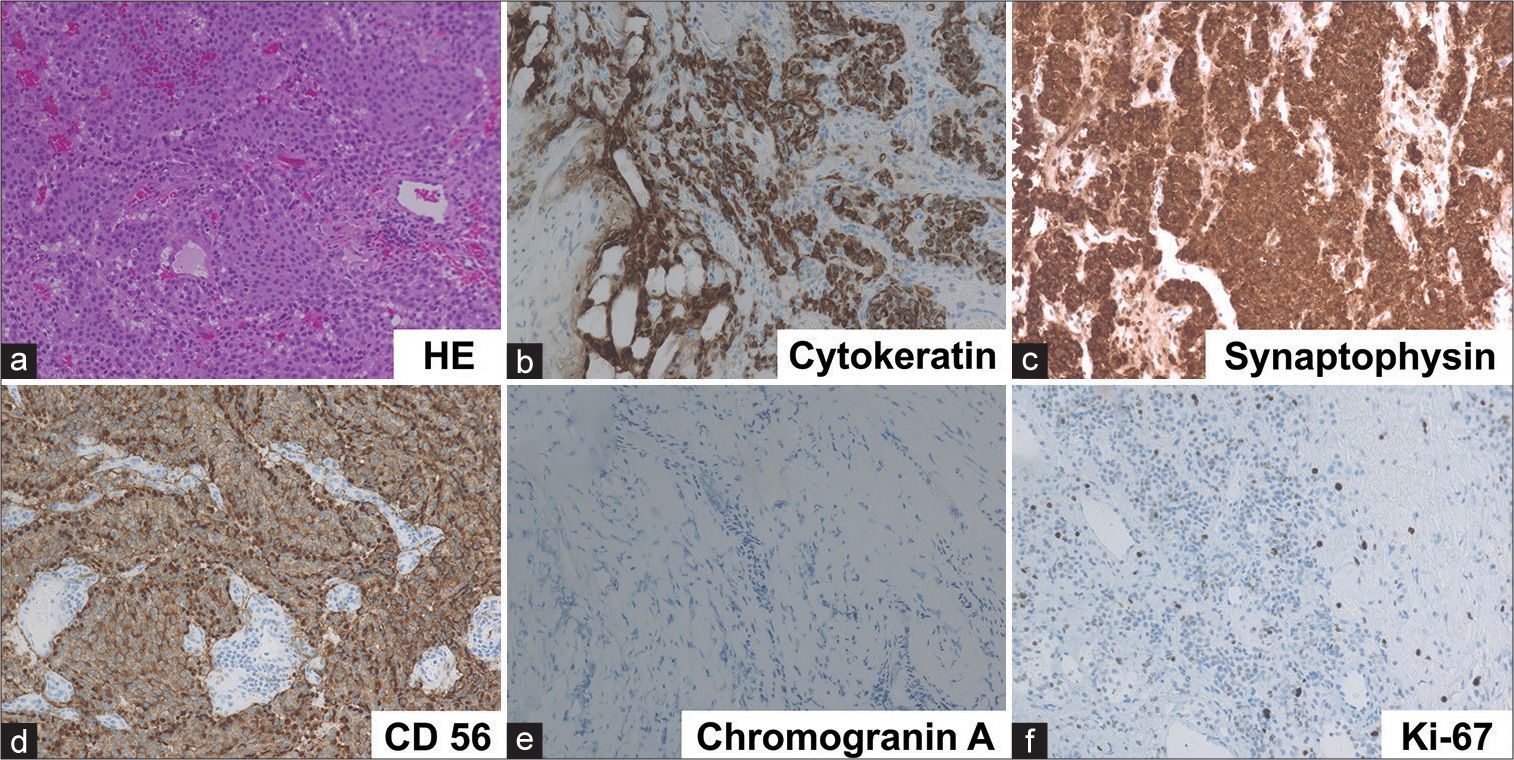
Figure 3:
Histological findings indicating metastatic neuroendocrine tumors. Hematoxylin and eosin staining showed monotonous tumor cells with round nuclei and eosinophilic cytoplasm arranged in nested or trabecular pattern (a). Immunohistochemically, tumor cells were positive for cytokeratin (b), synaptophysin (c), and CD56 (d), although they were negative for chromogranin A (e). Ki-67 labeling index was 5.7% (f).
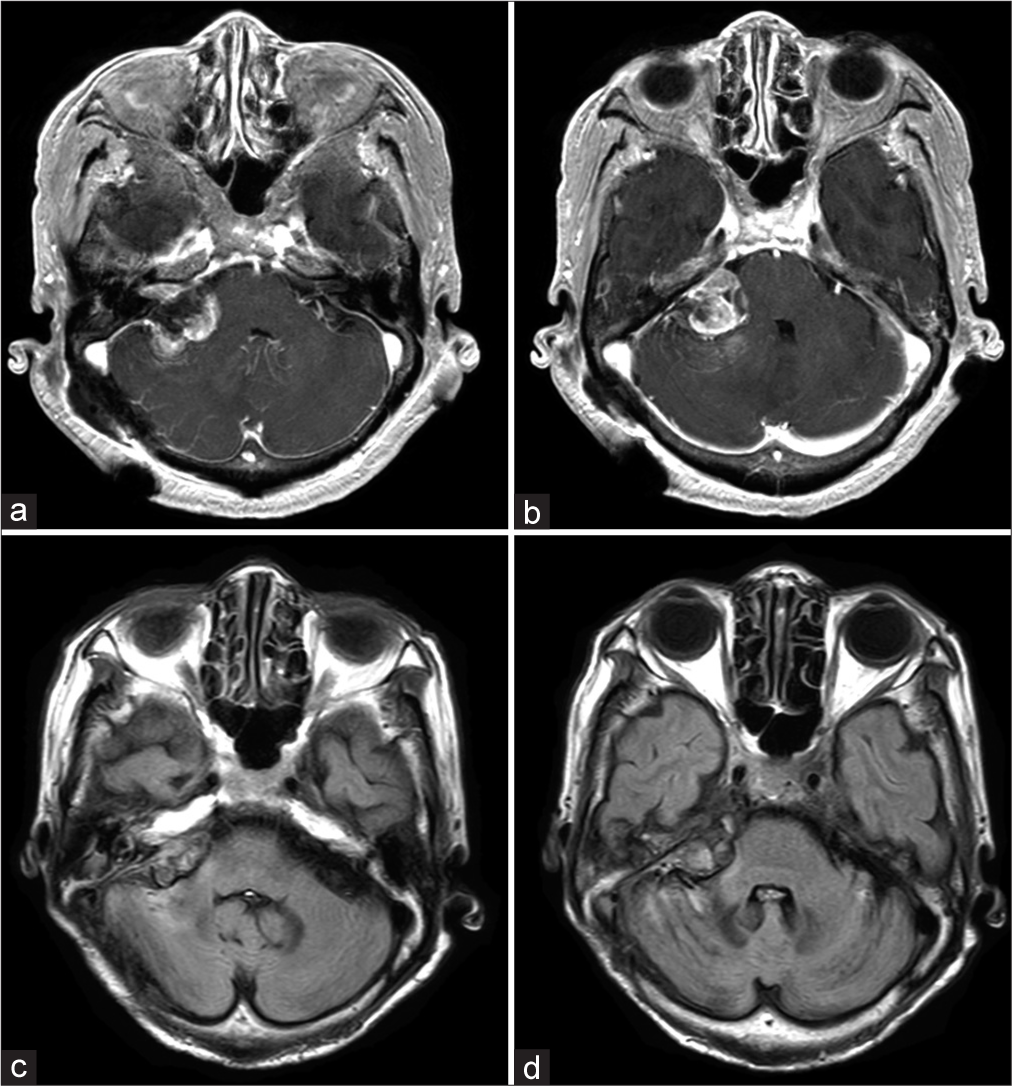
At 11 months of follow-up, the lesion was confirmed to have shrunk on MRI [
DISCUSSION AND CONCLUSION
NETs are a rare, heterogeneous group of neoplasms arising from neuroendocrine cells.[
The differential diagnosis of CPA tumors is diverse; the most common being vestibular schwannoma (70–90%), followed by meningioma (5–15%), epidermoid cyst (6%), and lipoma (1%).[
There is no standard treatment for intracranial metastases of NETs due to their rarity. Treatments include surgery, stereotactic radiosurgery (SRS), whole-brain radiation therapy (WBRT), and systemic chemotherapy, either alone or in combination.[
Brain metastases from NETs are highly vascular and the complication rates (13% mortality and 7% morbidity) of resection are reported to be higher than those of other tumors.[
Although WBRT after resection of carcinoid brain metastases has been reported to prolong the survival time,[
The 5-year survival rate for NETs and those with distant metastases excluding the brain are reported to be 60–67%[
We encountered a case of a primary retroperitoneal NET that metastasized to the CPA, initially resembling a vestibular schwannoma on imaging. Careful follow-up imaging and appropriate treatment strategies were useful to manage the metastasis. Although NETs metastasizing to the CPA are extremely rare, this possibility should be considered when patients with NETs have intracranial lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bhojwani N, Huang J, Gupta A, Badve C, Cohen ML, Wolansky LJ. Rectal carcinoid tumor metastasis to a skull base meningioma. Neuroradiol J. 2016. 29: 49-51
2. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017. 18: 1049-60
3. Cao F, Sada DM, Lai S, Sada YH. Stereotactic radiosurgery for carcinoid brain metastasis: A case report. Cureus. 2019. 11: e5509
4. Harrison CJ, Martin SC, Hofer M, Corkill R, Jeyaretna DS, Griffiths SJ. More than meets the MRI: Case report of a carcinoid tumour metastasis mimicking a meningioma. Br J Neurosurg. 2019. 33: 229-30
5. Hlatky R, Suki D, Sawaya R. Carcinoid metastasis to the brain. Cancer. 2004. 101: 2605-13
6. Huang PS, Oz M. Malignant carcinoid tumor metastatic to the dura mater simulating a meningioma. Neurosurgery. 1991. 29: 449-52
7. Inoue Y. Two cases of unusual cerebello-pontine angle tumor. Otol Japan. 2000. 10: 593-7
8. Kayama T, Sato S, Sakurada K, Mizusawa J, Nishikawa R, Narita Y. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): A phase III, noninferiority, randomized controlled trial. J Clin Oncol. 2018. p. JCO2018786186
9. Maiuri F, Cappabianca P, Del Basso De Caro M, Esposito F. Single brain metastases of carcinoid tumors. J Neurooncol. 2004. 66: 327-32
10. Mallory GW, Fang S, Giannini C, Van Gompel JJ, Parney IF. Brain carcinoid metastases: Outcomes and prognostic factors. J Neurosurg. 2013. 118: 889-95
11. Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005. 128: 1717-51
12. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003. 97: 934-59
13. Persad AR, Khormi YH, van Landeghem F, Chow MM. Unusual case of hemangioblastoma of the cerebellopontine angle. Surg Neurol Int. 2017. 8: 264
14. Porter DG, Chakrabarty A, McEvoy A, Bradford R. Intracranial carcinoid without evidence of extracranial disease. Neuropathol Appl Neurobiol. 2000. 26: 298-300
15. Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012. 61: 6-32
16. Reinas R, Santos RB, Kitumba D, Furtado A, Alves ÓL, Resende M. Posterior fossa metastasis of lung carcinoid tumor: Case report. Br J Neurosurg. 2021. 35: 364-6
17. Zeitels J, Naunheim K, Kaplan EL, Straus F. Carcinoid tumors: A 37-year experience. Arch Surg. 1982. 117: 732-7