- Department of Neurosurgery, University at Buffalo, Buffalo, New York, United States.
DOI:10.25259/SNI_593_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Steven B. Housley, Kunal Vakharia, Muhammad Waqas, Adnan H. Siddiqui. Cerebral hypoperfusion necessitating additional bypass following hunterian ligation of the internal carotid artery despite reassuring intraoperative challenges: Video case report. 20-Jan-2021;12:22
How to cite this URL: Steven B. Housley, Kunal Vakharia, Muhammad Waqas, Adnan H. Siddiqui. Cerebral hypoperfusion necessitating additional bypass following hunterian ligation of the internal carotid artery despite reassuring intraoperative challenges: Video case report. 20-Jan-2021;12:22. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10538
Abstract
Background: Hunterian ligation has been adapted for complex intracranial aneurysm repair when other, more modern techniques are insufficient. Before drastic alteration of cerebral blood flow dynamics, intraoperative challenges and consideration of blood flow dynamics must be completed to ensure adequate perfusion postligation. On satisfaction, ligation may proceed; however, subtle changes related to hypoperfusion may not be immediately observed during intraoperative challenge under general anesthesia and/or before onset of the vasospasm window.
Case Description: In this report, we describe a patient who presented with a Hunt-Hess Grade III subarachnoid hemorrhage (SAH), with a right internal carotid artery (ICA) occlusion and a ruptured giant left ICA aneurysm. Endovascular treatment of the aneurysm was aborted because the nominal, 9 mm diameter of the ICA was too large for any intracranial balloon or stent. Three days later, she underwent a left-sided “insurance” extracranial-tointracranial arterial bypass (EIAB) using the superficial temporal artery simultaneously with hunterian ligation of the left ICA following reassuring results on intraoperative occlusion challenge. Over several days, her neurologic condition declined concurrent with the vasospasm window, and a right-sided EIAB was required to augment vascular supply. Following a protracted hospital course, the patient became progressively more independent and is currently residing in an assisted living facility.
Conclusion: We illustrate an ultimately successful microsurgical treatment option in the setting of acute SAH that highlights the importance of cerebrovascular reserve and blood flow replacement in the setting of a compromised circle of Willis, especially during the vasospasm window.
Keywords: Extracranial-to-intracranial arterial bypass, Hunterian ligation, Hypoperfusion, Intracranial aneurysm, Microvascular neurosurgery, Subarachnoid hemorrhage
INTRODUCTION
Hunterian ligations were popularized in the 1800s by John Hunter who demonstrated a “safe and reproducible means of ligating peripheral arteries.”[
CASE DESCRIPTION
Our patient is a 71-year-old woman on aspirin (81 mg, daily) with a history of the right internal carotid artery (ICA) occlusion and a giant left cavernous carotid aneurysm that had been followed conservatively by an outside physician. She presented to an outside hospital with a thunderclap headache, nausea, vomiting, meningismus, and confusion consistent with Hunt-Hess Grade III SAH. She was transferred to our facility where noncontrast computed tomography (CT) revealed a Fisher Grade 4 SAH as well as bony erosion and intradural extension of the left cavernous carotid aneurysm [
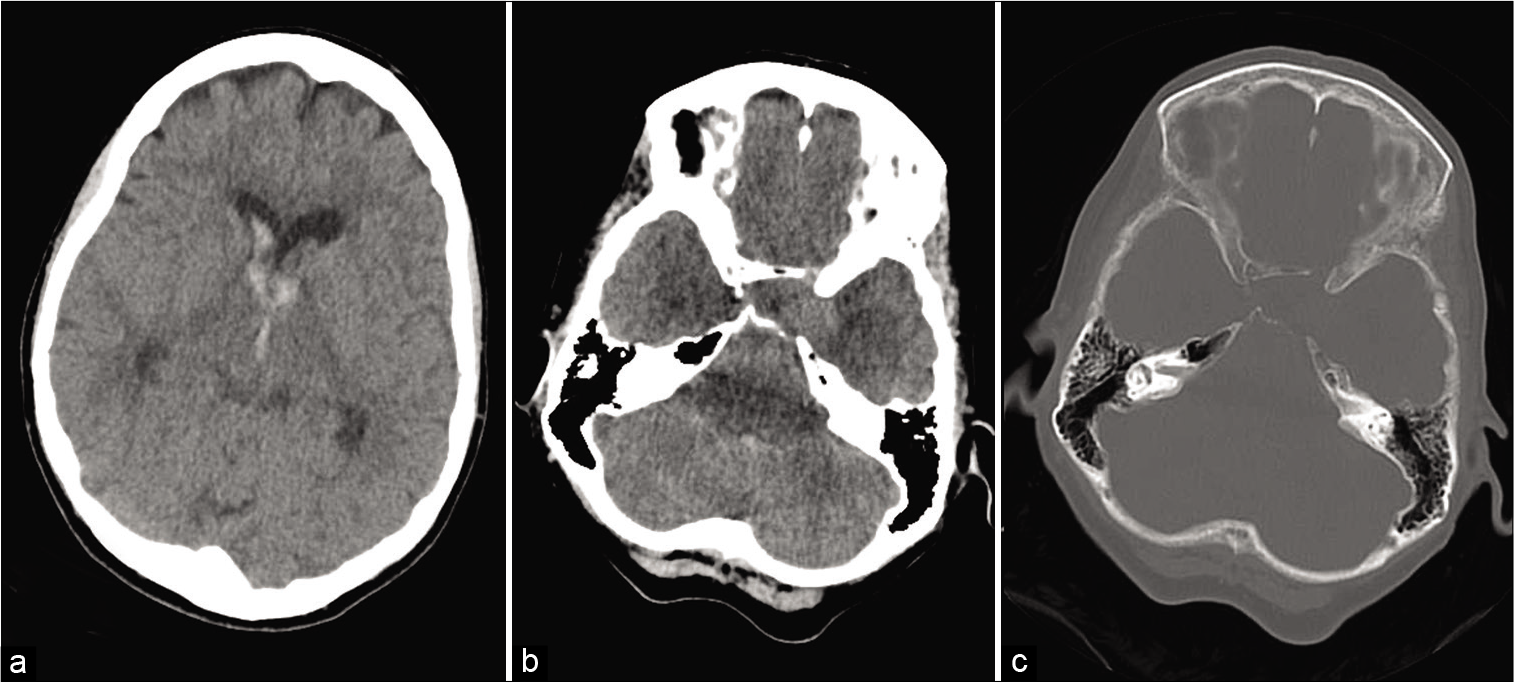
Figure 1:
Noncontrast computed tomography (CT) scan of the head showing intraventricular hemorrhage in the lateral and third ventricles (a); noncontrast CT scan showing left cavernous carotid aneurysm (b); bone window showing bony erosion in the left cavernous sinus secondary to cavernous carotid aneurysm (c).
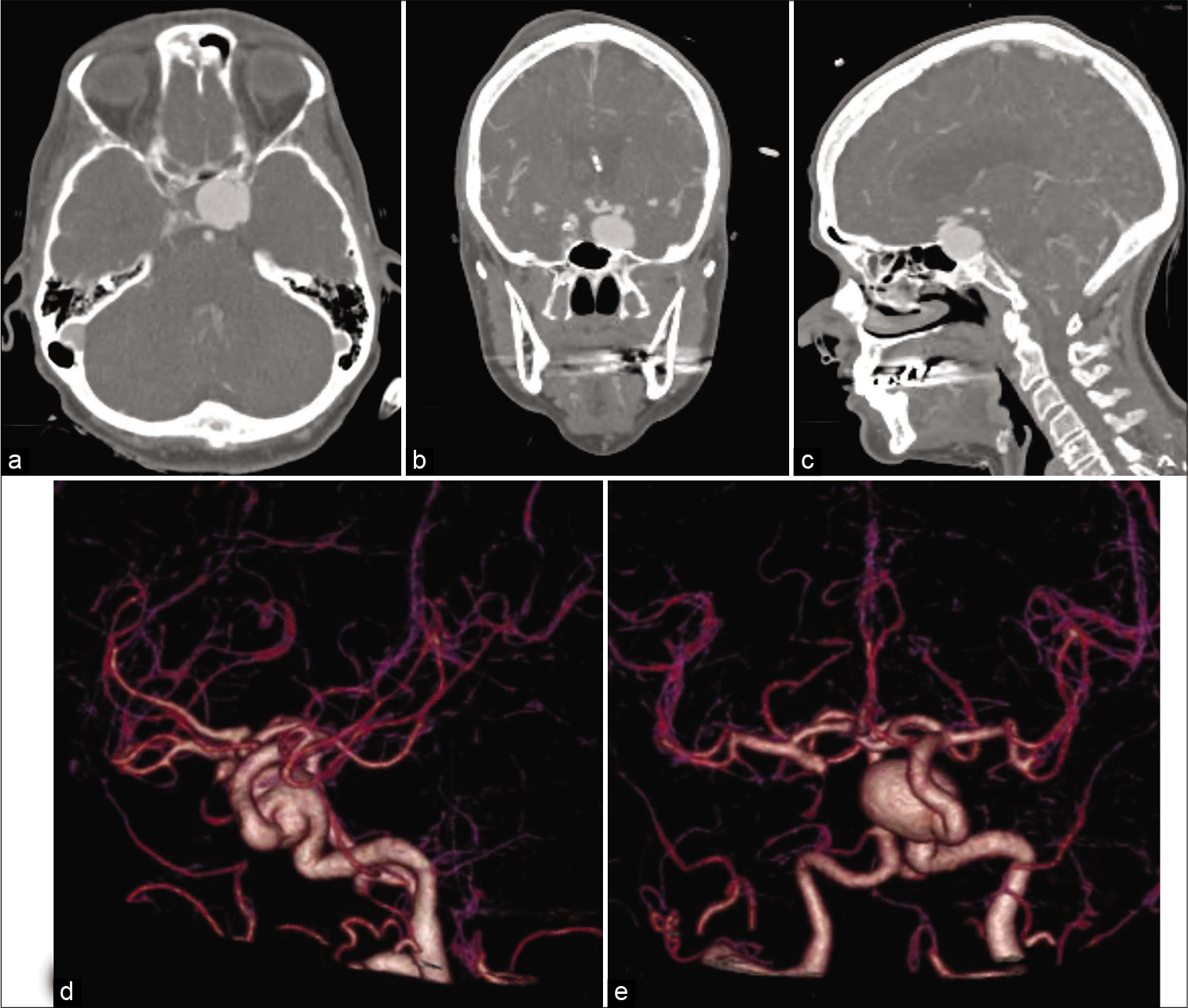
On hospital day (HD) 1, she was taken for a diagnostic cerebral angiogram (DSA) to further characterize her vascular lesions. A giant left cavernous carotid aneurysm measuring approximately 24×17 mm was observed. No other vascular lesions were revealed, and SAH protocol was continued. On HD 4, she was taken for Pipeline (Medtronic, Dublin, Ireland) embolization of the left cavernous ICA aneurysm. Flow diversion could not be performed because the nominal diameter of the petrocavernous carotid artery measured close to 9 mm, making it too large for any available adjunctive balloons or intracranial stents [
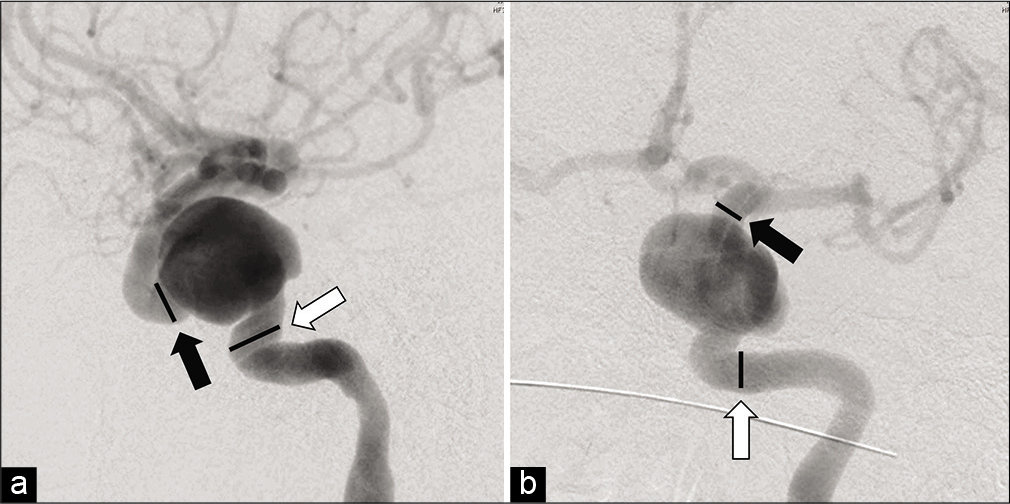
Figure 3:
Lateral digital subtraction angiographic image showing nominal diameters of the parent vessel of 8.67 mm (white arrow) and 6.67 mm (black arrow) (a); anteroposterior digital subtraction angiographic image showing nominal parent artery diameters of 6 mm (white arrow) and 5.1 mm (black arrow) (b).
Postoperatively, the patient was at her neurological baseline level, apart from trace weakness in her lower extremities. Through HD 7, the patient’s mentation slowly declined. Perfusion CT imaging demonstrated a lack of vascular reserve in the left hemisphere on acetazolamide challenge.[
On HD 8, a right-sided external carotid artery-to-MCA high-flow bypass using an interpositional radial artery graft was performed [
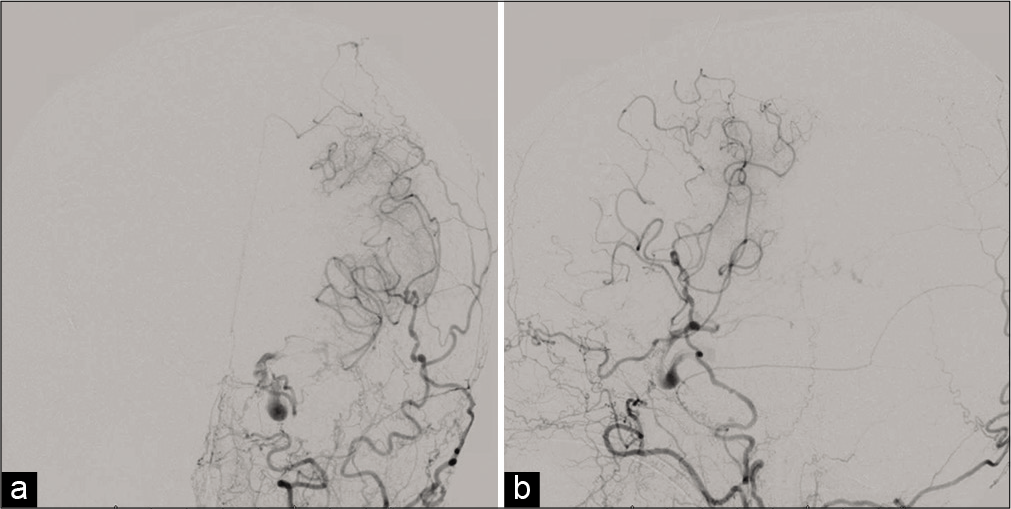
Postoperatively, the patient experienced a protracted hospital course with a poor neurological examination, despite only a few scattered areas of restricted diffusion in the left thalamus and bilateral watershed regions on magnetic resonance (MR) imaging. In addition, changes in flow dynamics consistent with patent bilateral bypasses were observed, including filling of the left-sided circulation from the right-sided high-flow bypass [
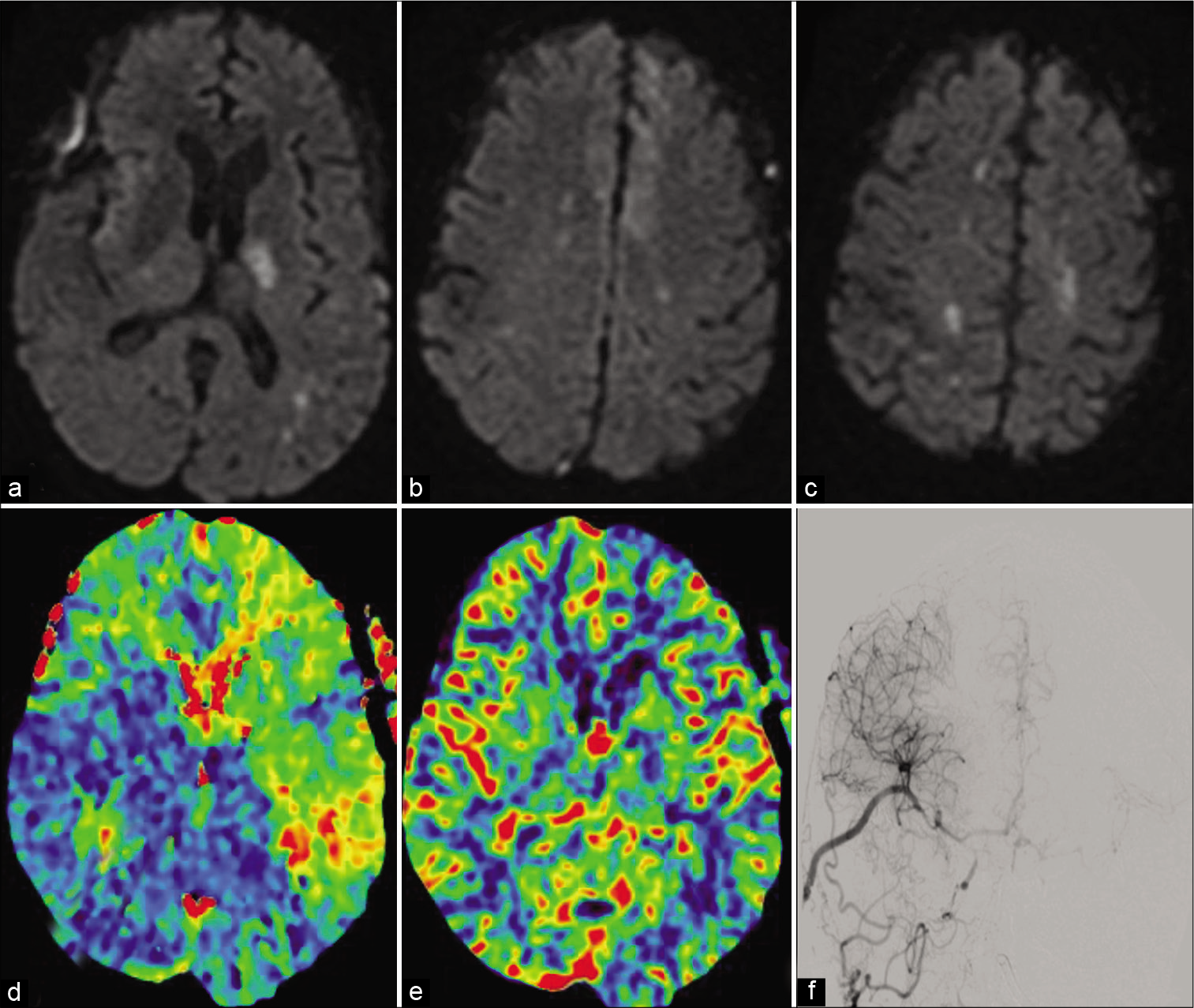
Figure 5:
Diffusion restriction imaging showing ischemic regions of the left thalamus, bilateral anterior cerebral artery territory, and bilateral watershed regions (a-c). Computed tomography perfusion imaging showing time to peak in the right cerebral circulation (d) with preserved cerebral blood volume (e). Diagnostic angiogram showing partial left anterior circulation filling from the right side (f).
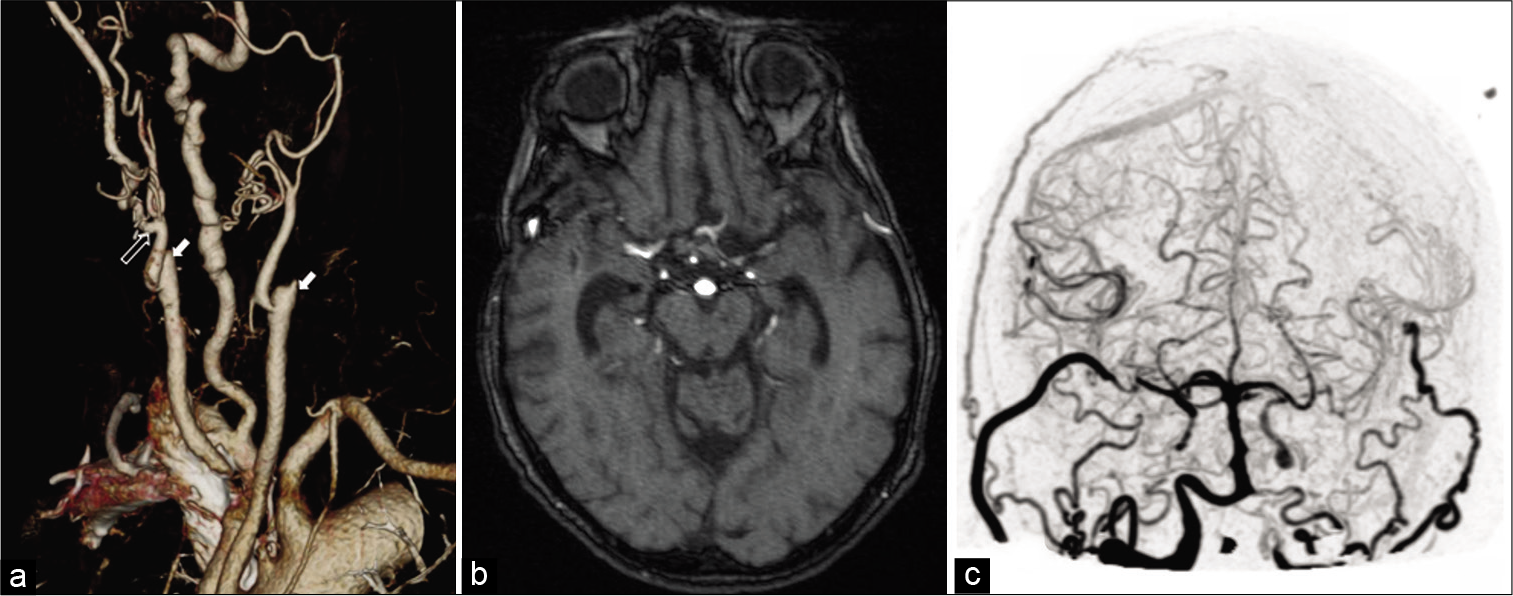
Figure 6:
3D reconstruction of CT of the neck showing bilateral carotid occlusions and patent proximal bypasses bilaterally at 7 months. White hollow arrow points to the proximal anastomosis of the radial artery interposition graft. Solid arrows indicate bilateral internal carotid artery occlusions. Large right dominant vertebral artery. (a) Magnetic resonance angiography at 7 months showing patency of the bypasses bilaterally (b); Immediate postoperative 4D CT angiography reconstruction showing bilateral patency of the bypasses (c).
DISCUSSION
Advancements in intracranial aneurysm treatments have expanded tremendously since the first reports of hunterian ligations; however, hunterian ligation remains the best option for a small subset of patients. Our patient had a ruptured giant left cavernous carotid aneurysm that was not amenable to endovascular intervention as well as a preexisting contralateral ICA occlusion that highlighted the importance of flow replacement during ligation of a major cerebral artery. This is especially true when factoring in the percentage of blood flow typically supplied by each major intracranial artery. In the typical intracranial circulation, average percentages of unilateral blood flow are 36% in the ICA and 14% in the vertebral artery;[
Video 1
Video Annotations
0:50 – Initial digital subtraction angiography findings 2:07 – Left bypass 4:35 – Hunterian ligation of the left internal carotid artery 5:25 – Perfusion deficits/worsening 5:35 – Right bypass 8:51 – Left side filling from the right bypass.
CONCLUSION
This case illustrates the importance of considering blood flow replacement when selecting whether to utilize low-flow versus high-flow bypass in procedures that require acute occlusion (hunterian ligation) of a major cerebral artery. In addition, it highlights the limitations of using intraoperative electrophysiologic monitoring to access collateral blood flow, especially when concurrent with the vasospasm window following acute SAH. Although hunterian ligation is reserved for cases with limited options due to an elevated risk profile, outcomes can be positive if managed appropriately. In patients with reduced cerebrovascular reserve, it is imperative that clinicians be able to recognize signs of hypoperfusion and be prepared to intervene appropriately to avoid irreversible ischemia and suboptimal outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Ameen AA, Illingworth R. Anti-fibrinolytic treatment in the pre-operative management of subarachnoid haemorrhage caused by ruptured intracranial aneurysm. J Neurol Neurosurg Psychiatry. 1981. 44: 220-6
2. Barnett DW, Barrow DL, Joseph GJ. Combined extracranialintracranial bypass and intraoperative balloon occlusion for the treatment of intracavernous and proximal carotid artery aneurysms. Neurosurgery. 1994. 35: 92-8
3. Morton RP, Moore AE, Barber J, Tariq F, Hare K, Ghodke B. Monitoring flow in extracranial-intracranial bypass grafts using duplex ultrasonography: A single-center experience in 80 grafts over 8 years. Neurosurgery. 2014. 74: 62-70
4. Polevaya NV, Kalani MY, Steinberg GK, Tse VC. The transition from hunterian ligation to intracranial aneurysm clips: A historical perspective. Neurosurg Focus. 2006. 20: E3
5. Sia SF, Morgan MK. High flow extracranial-to-intracranial brain bypass surgery. J Clin Neurosci. 2013. 20: 1-5
6. Starke RM, Connolly ES. Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Rebleeding after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011. 15: 241-6
7. Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: Techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol. 2009. 30: 876-84
8. Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2015. 35: 648-54