- Department of Neurosurgery, Sendai City Hospital, Sendai, Miyagi, Japan.
- Department of Neurosurgery, Isinomaki Red Cross Hospital, Ishinomaki, Japan.
- Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan.
Correspondence Address:
Arata Nagai, Department of Neurosurgery, Sendai City Hospital, Sendai, Miyagi, Japan.
DOI:10.25259/SNI_358_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Arata Nagai1, Hiroshi Karibe1, Ayumi Narisawa1, Motonobu Kameyama1, Shuichi Ishikawa2, Naoya Iwabuchi2, Teiji Tominaga3. Cerebral infarction following administration of andexanet alfa for anticoagulant reversal in a patient with traumatic acute subdural hematoma. 11-Aug-2023;14:286
How to cite this URL: Arata Nagai1, Hiroshi Karibe1, Ayumi Narisawa1, Motonobu Kameyama1, Shuichi Ishikawa2, Naoya Iwabuchi2, Teiji Tominaga3. Cerebral infarction following administration of andexanet alfa for anticoagulant reversal in a patient with traumatic acute subdural hematoma. 11-Aug-2023;14:286. Available from: https://surgicalneurologyint.com/surgicalint-articles/12494/
Abstract
Background: Anticoagulants prevent thrombosis in patients with atrial fibrillation (AF) and venous thromboembolism but increase the risk of hemorrhagic complications. If severe bleeding occurs with anticoagulant use, discontinuation and rapid reversal are essential. However, the optimal timing for resuming anticoagulants after using reversal agents remains unclear. Here, we report early cerebral infarction following the use of andexanet alfa (AA), a specific reversal agent for factor Xa inhibitors, in a patient with traumatic acute subdural hematoma (ASDH). The possible causes of thromboembolic complication and the optimal timing for anticoagulant resumption are discussed.
Case Description: An 84-year-old woman receiving rivaroxaban for AF presented with impaired consciousness after a head injury. Computed tomography (CT) revealed right ASDH. The patient was administered AA and underwent craniotomy. Although the hematoma was entirely removed, she developed multiple cerebral infarctions 10 h after the surgery. These infarctions were considered cardiogenic cerebral embolisms and rivaroxaban was therefore resumed on the same day. This case indicates the possibility of early cerebral infarction after using a specific reversal agent for factor Xa inhibitors.
Conclusion: Most studies suggest that the safest time for resuming anticoagulants after using reversal agents is between 7 and 12 days. The present case showed that embolic complications may develop much earlier than expected. Early readministration of anticoagulant may allow for adequate prevention of the acute thrombotic syndromes.
Keywords: Acute subdural hematoma, Andexanet alfa, Anticoagulant, Cerebral infarction
INTRODUCTION
Direct oral anticoagulants (DOAC), including factor Xa inhibitors, reduce the incidence of thromboembolic events in patients with atrial fibrillation (AF) or venous thromboembolism.[
While the efficacy of such reversal agents is being established, the timing of anticoagulant therapy resumption remains controversial. After reversal of anticoagulant therapy, the risk of thromboembolic events increases with delays in anticoagulant resumption. Clinicians must make the difficult decision regarding when to resume anticoagulants.
Anticoagulant-associated traumatic acute subdural hematoma (ASDH) is a devastating injury with high morbidity and mortality.[
In this report, we present a case of traumatic ASDH during anticoagulation therapy that resulted in early cerebral infarction after AA administration. We also discuss the timing of anticoagulant resumption in patients with traumatic ASDH who received anticoagulant reversal agents. The study participant provided informed consent and the study design was approved by the appropriate Ethics Review Board.
CASE REPORT
An 84-year-old woman with a medical history of AF, hypertension, diabetes mellitus, and cerebral infarction and who was receiving rivaroxaban (15 mg/day) experienced frequent falls in her residential facility due to delirium. She then developed a consciousness disorder on awakening. She was transferred to our hospital with a diagnosis of the right ASDH [
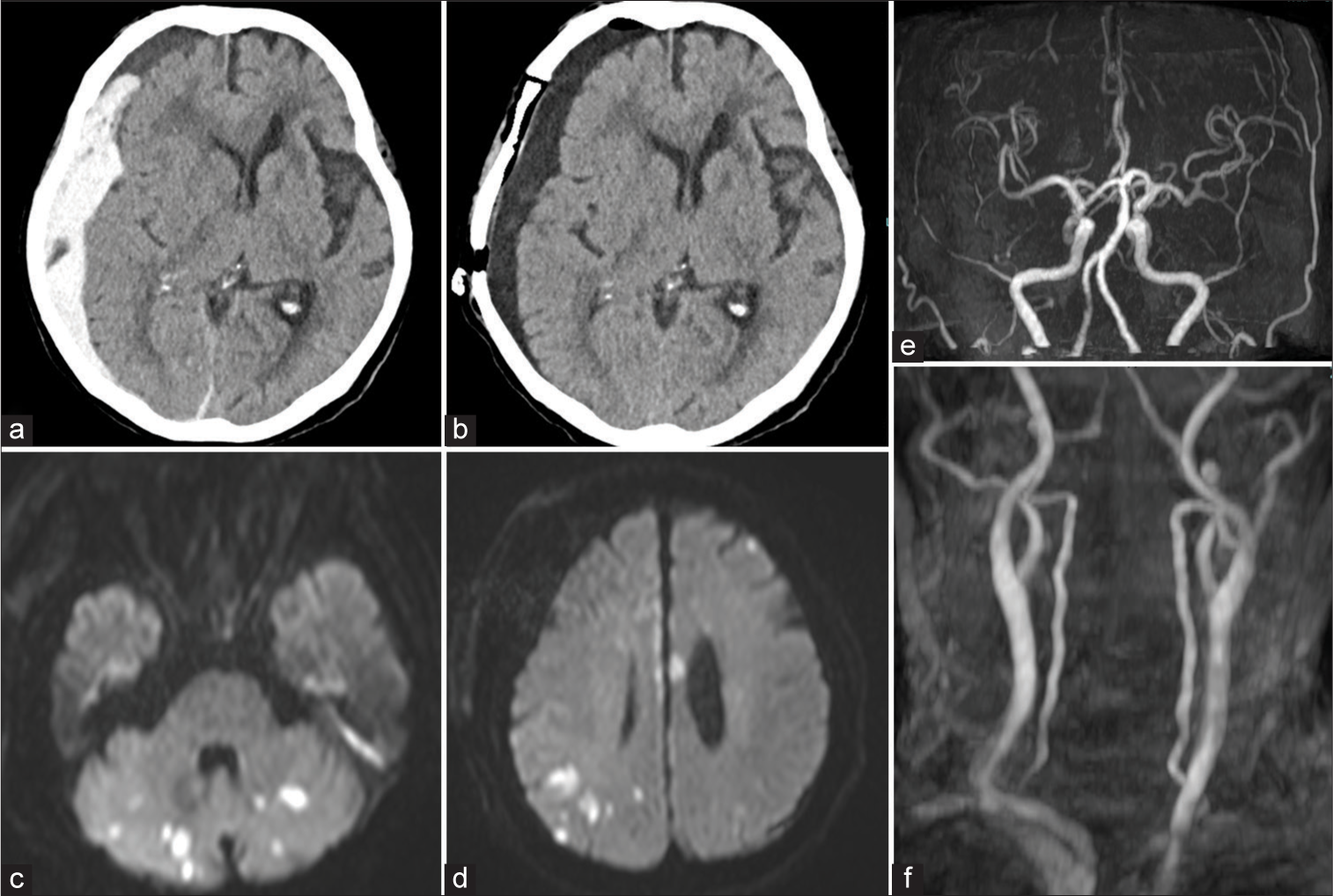
Figure 1:
Neuroradiological findings of the case. (a) Plain computed tomography (CT) taken at admission showing a right-sided acute subdural hematoma with low-density areas in some places. (b) CT immediately after surgery showing total subdural hematoma removal and effective intracranial decompression. (c and d) Magnetic resonance imaging taken the day after surgery showing multiple acute cerebral infarctions in the bilateral cerebellar hemispheres, corpus callosum, and cerebral cortex. (e and f) Magnetic resonance angiography showed no vascular abnormalities such as cerebral vascular stenosis, occlusion, or vasospasm.
DISCUSSION
Anticoagulant therapy is effective in preventing ischemic disease in patients with AF, mechanical cardiac valve replacement, or deep venous thrombosis. In contrast, it increases the risk of poor outcomes in patients with traumatic ASDH, mainly resulting from the size or delayed enlargement of the hematoma.[
On the other hand, discontinuation and/or reversal of anticoagulants may increase the risk of thromboembolic complications. In the present case, cardiogenic embolic infarctions occurred within a day after reversal of anticoagulation with AA. The mechanism of thromboembolic complications may be related to the following factors: (1) characteristics of the reversal agent itself, (2) underlying personal health condition, and (3) pathogenesis of the hemorrhage.
Thromboembolic risk related to characteristics of the reversal agent
It is believed that AA does not exert procoagulant effects. It is unable to cleave prothrombin into thrombin because the serine in the active site is replaced by alanine.[
Thromboembolic risk related to underlying disease
The risk of thromboembolic complications may be different in each patient depending on the underlying disease for which anticoagulation is introduced. It is suggested that anticoagulants should be resumed earlier than previously thought, approximately 3 days after medical presentation.[
Thromboembolic risk related to the pathogenesis of the hemorrhage
The patient had sustained traumatic ASDH. The coagulation/ fibrinolytic system may be abnormal in traumatic hemorrhage compared with nontraumatic hemorrhage, resulting in postoperative hypercoagulability.[
PTCI of the occipital lobe is well described and often results from compression of the posterior cerebral artery against the tentorium by the herniating medial temporal lobe. Middle cerebral artery territory infarcts may occur as a result of displacement from mass lesions and herniation as well. Cerebral infarction in this case did not depend on the area of vascular control; the multiple small infarcts suggest a cause other than cerebral herniation. Other mechanisms may include vasospasm or thromboembolic events from injured carotid and vertebral arteries. However, magnetic resonance angiography demonstrated no concomitant vascular injury in this patient. Based on the above, the discontinuation of anticoagulants and use of the reversal agent were likely involved in the embolism in this case.
Appropriate time to resume anticoagulants
It appears safe to discontinue anticoagulation for brief periods in nontraumatic anticoagulant-related cerebral hemorrhage.[
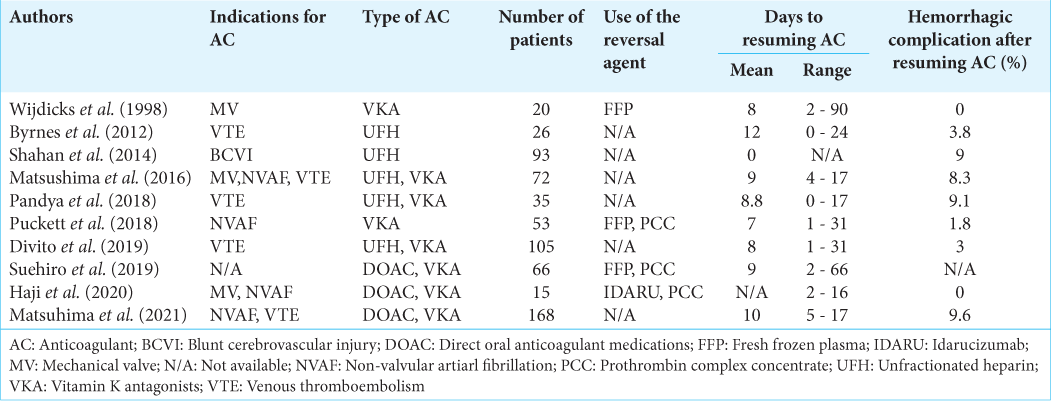
Three of the reports summarized in
There have been some reports of heparin initiation in the hyperacute phase within 24 h after injury for deep vein thrombosis prevention.[
As observed in the present case, cerebral infarction sometimes occurs early after reversal agent use. A meta-analysis of taking anticoagulants showed all thromboembolic complication occurred within a few days (1–4 days) of reversal agent administration.[
CONCLUSION
AA was used as an effective reversal agent to achieve hemostasis in a patient with traumatic ASDH. The patient had thromboembolic events, and it cannot be ruled out that AA may have been a contributing factor to their occurrence. In patients with traumatic ASDH treated with AA, early anticoagulant resumption may need to be considered because of the higher embolization risk. More real-world studies on AA use, including the timing of anticoagulant resumption, will greatly add to our knowledge and comfort in using this important drug.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abuan I, Wong KH, Bolinske B, Hale KS. Andexanet alfa: A recombinant modified human factor Xa protein for drug reversal of rivaroxaban and apixaban. J Pharm Technol. 2019. 35: 119-25
2. Batchelor JS, Grayson A. A meta-analysis to determine the effect of anticoagulation on mortality in patients with blunt head trauma. Br J Neurosurg. 2012. 26: 525-30
3. Becattini C, Franco L, Beyer-Westendorf J, Masotti L, Nitti C, Vanni S. Major bleeding with vitamin K antagonists or direct oral anticoagulants in real-life. Int J Cardiol. 2017. 227: 261-6
4. Byrnes MC, Irwin E, Roach R, James M, Horst PK, Reicks P. Therapeutic anticoagulation can be safely accomplished in selected patients with traumatic intracranial hemorrhage. World J Emerg Surg. 2012. 7: 25
5. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH. Full study reort of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019. 380: 1326-35
6. Demchuk AM, Yue P, Zotova E, Nakamya J, Xu L, Milling TJ. Hemostatic efficacy and anti-FXa (factor Xa) reversal with andexanet alfa in intracranial hemorrhage: ANNEXA-4 substudy. Stroke. 2021. 52: 2096-105
7. Divito A, Kerr K, Wilkerson C, Shepard S, Choi A, Kitagawa RS. Use of anticoagulation agents after traumatic intracranial hemorrhage. World Neurosurg. 2019. 123: 25-30
8. Dossett LA, Riesel JN, Griffin MR, Cotton BA. Prevalence and implications of preinjury warfarin use: An analysis of the National Trauma Databank. Arch Surg. 2011. 146: 565-70
9. Epstein DS, Mitra B, O’Reilly G, Rosenfeld JV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: A systematic review and meta-analysis. Injury. 2014. 45: 819-24
10. Francesco D, Chiara M, Matteo GP, Mark C, David G, Elaine H. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of viamin K antagonists. Thromb Haemost. 2011. 106: 429-38
11. Franko J, Kish KJ, O’Connell BG, Subramanian S, Yuschak JV. Advanced age and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 2006. 61: 107-10
12. Gómez-Outes A, Alcubilla P, Calvo-Rojas G, TerleiraFernández AI, Suárez-Gea ML, Lecumberri R. Meta-analysis of reversal agents for severe bleeding associated with direct oral anticoagulants. J Am Coll Cardiol. 2021. 77: 2987-3001
13. Goodnignt SH, Kenoyer G, Rapaport SI, Patch MJ, Lee JA, Kurze T. Defibrination after brain-tissue destrution: A serious complication of head injury. N Engl J Med. 1974. 290: 1043-7
14. Grigorian A, Kabutey NK, Schubl S, de Virgilio C, Joe V, Dolich M. Blunt cerebrovascular injury incidence, strokerate, and mortality with the expanded Denver criteria. Surgery (United States). 2018. 164: 494-9
15. Haji K, Suehiro E, Kiyohira M, Hujiyama Y, Suzuki M. Effect of antithrombotic drugs reversal on geriatric traumatic brain injury Japan. No Shinkei Geka. 2020. 48: 497-504
16. Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien). 2008. 150: 165-75
17. Hawryluk GW, Austin JW, Furlan JC, Lee JB, O’Kelly C, Fehlings MG. Management of anticoagulation following central nervous system hemorrhage in patients with high thromboembolic risk. J Thromb Haemost. 2010. 8: 1500-8
18. Inamasu J, Nakatsukasa M, Kuramae T, Nakagawa Y, Miyatake S, Tomiyasu K. Influence of age and anti-platelet/ anti-coagulant use on the outcome of elderly patients with fall-related traumatic intracranial hemorrhage. Neurol Med Chir (Tokyo). 2010. 50: 1051-5
19. Itshayek E, Rosenthal G, Fraifeld S, Perez-Sanchez X, Cohen JE, Spektor S. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006. 58: 851-6
20. Karibe H, Hayashi T, Narisawa A, Kameyama M, Nakagawa A, Tominaga T. Clinical characteristics and outcome in elderly patients with traumatic brain injury: For establishment of management strategy. Neurol Med Chir (Tokyo). 2017. 57: 418-25
21. Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015. 313: 824-36
22. Kuramatsu JB, Sembill JA, Gerner ST, Sprügel MI, Hagen M, Roeder SS. Management of therapeutic anticoagulation in patients with intracerebral haemorrhage and mechanical heart valves. Eur Heart J. 2018. 39: 1709-23
23. Lavoie A, Ratte S, Clas D, Demers J, Moore L, Martin M. Preinjury warfarin use among elderly patients with closed head injuries in a trauma center. J Trauma. 2004. 56: 802-7
24. Lu G, Hollenbach SJ, Baker DC, Tan S, Hutchaleelaha A, Curnutte JT. Preclinical safety and efficacy of andexanet alfa in animal models. J Thromb Haemost. 2017. 15: 1747-56
25. Maeda K, Koga M, Okada Y, Kimura K, Yamagami H, Okuda S. Nationwide survey of neuro-specialists’ opinions on anticoagulant therapy after intracerebral hemorrhage in patients with atrial fibrillation. J Neurol Sci. 2012. 312: 82-5
26. Matsushima K, Inaba K, Cho J, Mohammed H, Herr K, Leichtle S. Therapeutic anticoagulation in patients with traumatic brain injury. J Surg Res. 2016. 205: 186-91
27. Milling TJ, Refaai MA, Goldstein JN, Schneider A, Omert L, Amy H. Thromboembolic events after vitamin K antagonist reversal with 4-factor prothrombin complex concentrate: Exploratory analyses of two randomized, plasma-controlled studies. Ann Emerg Med. 2016. 67: 96-105
28. Milling TJ, Warach S, Johnston SC, Gajewski B, Costantini T, Price M. Restart TICrH: An adaptive randomized trial of time intervals to restart direct oral anticoagulants after traumatic intracranial hemorrhage. J Neurotrauma. 2021. 38: 1791-8
29. Mina AA, Knipfer JF, Park DY, Bair HA, Howells GA, Bendick PJ. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. 2002. 53: 668-72
30. Murthy SB, Gupta A, Merkler AE, Navi BB, Mandava P, Iadecola C. Restarting anticoagulant therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017. 48: 1594-600
31. Nielsen PB, Larsen TB, Skjøth F, Lip GY. Outcomes associated with resuming warfarin treatment after hemorrhagic stroke or traumatic intracranial hemorrhage in patients with atrial fibrillation. JAMA Intern Med. 2017. 177: 563-70
32. Osaki M, Koga M, Maeda K, Hasegawa Y, Nakagawara J, Furui E. A multicenter, prospective, observational study of warfarin-associated intracerebral hemorrhage: The SAMURAI-WAICH study. J Neurol Sci. 2015. 359: 72-7
33. Pandya U, Pattison J, Karas C, O’Mara M. Does the presence of subdural hemorrhage increase the risk of intracranial hemorrhage expansion after the initiation of antithrombotic medication?. Am Surg. 2018. 84: 416-21
34. Pennlert J, Overholser R, Asplund K, Carlberg B, Van Rompaye B, Wiklund PG. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017. 48: 314-20
35. Prexl O, Bruckbauer M, Voelckel W, Grottke O, Ponschab M, Maegele M. The impact of direct oral anticoagulants in traumatic brain injury patients greater than 60-years-old. Scand J Trauma Resusc Emerg Med. 2018. 26: 20
36. Puckett Y, Zhang K, Blasingame J, Lorenzana J, Parameswaran S, Brooks MD. Safest time to resume oral anticoagulation in patients with traumatic brain injury. Cureus. 2018. 10: e2920
37. Sakamoto Y, Nito C, Nishiyama Y, Suda S, Matsumoto N, Aoki J. Safety of anticoagulant therapy including direct oral anticoagulants in patients with acute spontaneous intracerebral hemorrhage. Circ J. 2019. 83: 441-6
38. Sartori M, Cosmi B. Andexanet alfa to reverse the anticoagulant activity of factor XA inhibitors: A review of design, development and potential place in therapy. J Thromb Thrombolysis. 2018. 45: 345-52
39. Shahan CP, Magnotti LJ, McBeth PB, Weinberg JA, Croce MA, Fabian TC. Early antithrombotic therapy is safe and effective in patients with blunt cerebrovascular injury and solid organ injury or traumatic brain injury. J Trauma Acute Care Surg. 2016. 81: 173-7
40. Staerk L, Fosbøl EL, Lamberts M, Bonde AN, Gadsbøll K, Sindet-Pedersen C. Resumption of oral anticoagulation following traumatic injury and risk of stroke and bleeding in patients with atrial fibrillation: A nationwide cohort study. Eur Heart J. 2018. 39: 1698-705
41. Suehiro E, Fujiyama Y, Kiyohira M, Haji K, Ishihara H, Nomura S. Risk of deterioration of geriatric traumatic brain injury in patients treated with antithrombotic drugs. World Neurosurg. 2019. 127: e1221-7
42. Suehiro E, Ishihara H, Fujiyama Y, Kiyohira M, Haji K, Nomura S. Corresponding to geriatric traumatic brain injury in patients taking antithrombotic agent. Jpn J Neurosurg. 2019. 28: 614-20
43. Tawil I, Stein DM, Mirvis SE, Scalea TM. Posttraumatic cerebral infarction: Incidence, outcome, and risk factors. J Trauma. 2008. 64: 849-53
44. Tierney KJ, Nayak NV, Prestigiacomo CJ, Sifri ZC. Neurosurgical intervention in patients with mild traumatic brain injury and its effect on neurological outcomes. J Neurosurg. 2016. 124: 538-45
45. Toyoda K, Yasaka M, Iwade K, Nagata K, Koretsune Y, Sakamoto T. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: A prospective, multicenter, observational study. Stroke. 2008. 39: 1740-5
46. Van Den Brand CL, Tolido T, Rambach AH, Hunink MG, Patka P, Jellema K. Systematic review and meta-analysis: Is pre-injury antiplatelet therapy associated with traumatic intracranial hemorrhage?. J Neurotrauma. 2017. 34: 1-7
47. Wafaisade A, Lefering R, Tjardes T, Wutzler S, Simanski C, Paffrath T. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit Care. 2010. 12: 211-9
48. Wijdicks EF, Schievink WI, Brown RD, Mullany CJ. The dilemma of discontinuation of anticoagulation therapy for patients with intracranial hemorrhage and mechanical heart valves. Neurosurgery. 1998. 42: 769-73
49. Wu YG, Chao Y, Gao G, Bao D, Dong Y, Wei X. Risk factors for cerebral infarction after moderate or severe traumatic brain injury. Ther Clin Risk Manag. 2021. 17: 433-40
50. Zhang J, Zhang F, Dong JF. Coagulopathy induced by traumatic brain injury: Systemic manifestation of a localized injury. Blood. 2018. 131: 2001-6