- Department of Neurological Surgery, University of California (UC) Irvine Medical Center, Orange, United States
- Keck School of Medicine of USC, University of Southern California, Los Angeles, United States
- Department of Neurology, University of California Irvine Medical Center, Orange, California, United States.
Correspondence Address:
Ishan Shah, Department of Neurological Surgery, University of California (UC) Irvine Medical Center, Orange, California, United States.
DOI:10.25259/SNI_679_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ishan Shah1,2, Patrick M. Chen3, Diem Kieu Thi Tran1, Jefferson W. Chen1. Cerebral microdialysis demonstrates improvements in brain metabolism with cerebrospinal fluid diversion in spontaneous intracerebral hemorrhage. 10-Nov-2023;14:395
How to cite this URL: Ishan Shah1,2, Patrick M. Chen3, Diem Kieu Thi Tran1, Jefferson W. Chen1. Cerebral microdialysis demonstrates improvements in brain metabolism with cerebrospinal fluid diversion in spontaneous intracerebral hemorrhage. 10-Nov-2023;14:395. Available from: https://surgicalneurologyint.com/surgicalint-articles/12631/
Abstract
Background: Cerebral microdialysis (CMD) is an FDA-approved multimodal invasive monitoring technique that provides local brain metabolism measurements through continuous interstitial brain fluid sampling at the bedside. The past applications in traumatic brain injury and subarachnoid hemorrhage show that acute brain injury (ABI) can lead to a metabolic crisis reflected by changes in cerebral glucose, pyruvate, and lactate. However, limited literature exists on CMD in spontaneous intracerebral hemorrhage (ICH).
Case Description: A 45-year-old woman presented with a Glasgow Coma Scale of 8T and left frontal ICH with a 6 mm midline shift. She underwent craniotomy and ICH evacuation. Intraoperatively, CMD, brain tissue oxygenation (PbtO2), intracranial pressure (ICP), and cerebral blood flow (CBF) catheters were placed, targeted toward the peri-hematoma region. Postoperatively, ICP was normal; however, PbtO2, CBF, glucose, and lactate/ pyruvate ratio were abnormal. Due to concern for the metabolic crisis, poor examination, and hydrocephalus on computed tomography of the head (CTH), she underwent external ventricular drainage (EVD). Post-EVD, all parameters normalized (P t-test). Monitors were removed, and she was discharged to a nursing facility with a modified Rankin scale of 4.
Conclusion: Here, we demonstrate the safe implementation of CMD in ICH and the use of CMD in tandem with PbtO2/ICP/CBF to guide treatment in ICH. Despite a normal ICP, numerous cerebral metabolic derangements existed and improved after cerebrospinal fluid diversion. A normal ICP may not reflect underlying metabolic-substrate demands of the brain during ABI. CMD and PbtO2/CBF monitoring augment traditional ICP monitoring in brain injury. Further prospective studies will be needed to understand further the interplay between ICP, PbtO2, CBF, and CMD values in ABI.
Keywords: Brain metabolism, Brain path, Cerebral brain oxygenation, Cerebral microdialysis, Spontaneous intracerebral hemorrhage
INTRODUCTION
Cerebral microdialysis (CMD) is a well-established, FDA-approved technique of multimodal brain monitoring used to provide measurements of local brain metabolism through continuous sampling of brain interstitial fluid. A catheter is inserted into the area of interest in the brain, yielding measurements of various metabolites such as glucose, lactate, pyruvate, and a lactate to pyruvate (L/P) ratio.[
Changes in metabolic activity relating to ICH are observed, particularly in the peri-hemorrhagic zone (PHZ), the area surrounding the point of injury or damage. Past studies on TBI and SAH have observed decreased glucose and elevated L/P from CMD catheters in the PHZ as compared to normal brain in the same patient, indicating an interruption of adequate metabolism in specific areas of the brain.[
Here, we describe a case of sICH that utilized CMD in tandem with PbtO2 and cerebral blood flow (CBF) monitoring to guide the initiation of external ventricular drainage (EVD) following craniotomy and minimally invasive parafascicular surgery for the evacuation of the hemorrhage.
CASE PRESENTATION
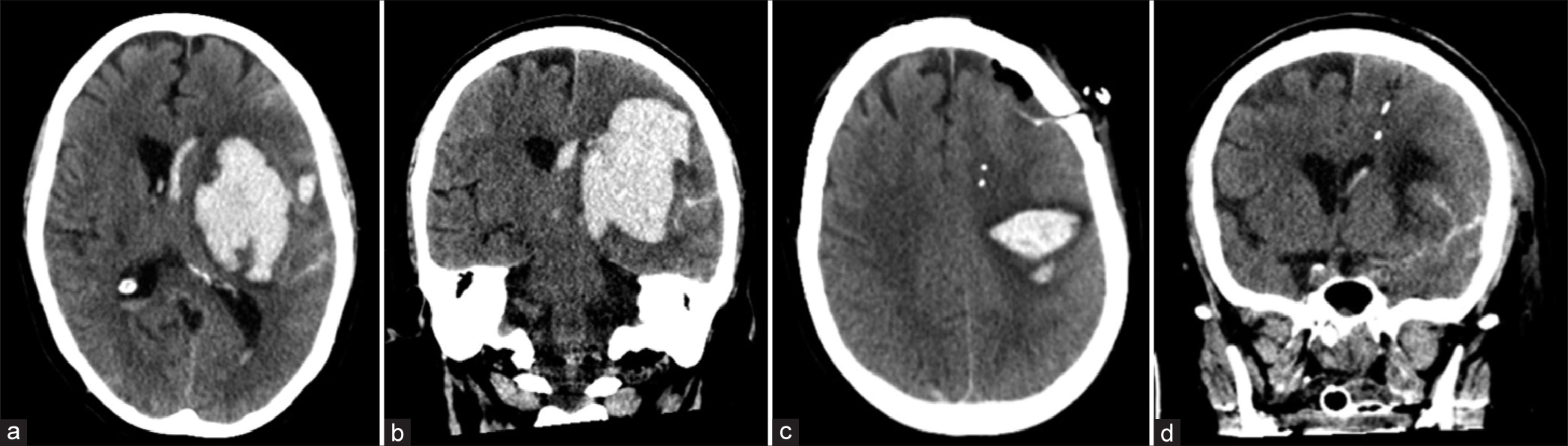
A 45-year-old female presented with slurred speech and left-sided twitching and weakness with rapid decline to becoming unresponsive. She had a past medical history of methamphetamine abuse and hypertension. Physical examination showed an intubated patient with a Glasgow Coma Score of 8T. She opened her eyes to noxious stimulation and had reactive pupils and purposeful movements of her left upper and lower extremities with minimal movements of the right upper and lower extremities. Computed tomography (CT) scans of the brain revealed an 87 cc (6.5 × 6.2 × 4.3 cm/2 using the A × B × C/2 estimation) ICH in the left frontal region (extending to the basal ganglia with intraventricular hemorrhage and effacement of the ventricles [
Figure 1:
Computed tomography scans. (a) Axial plane view of the brain 10 h before initial surgical evacuation of the intracerebral hemorrhage (ICH). (b) Coronal plane view of the brain 10 h before initial surgical evacuation of the ICH. (c) Axial plane view of the brain 28 h after surgical evacuation of the ICH. (d) Coronal plane view of the brain 28 h after surgical evacuation of the ICH.
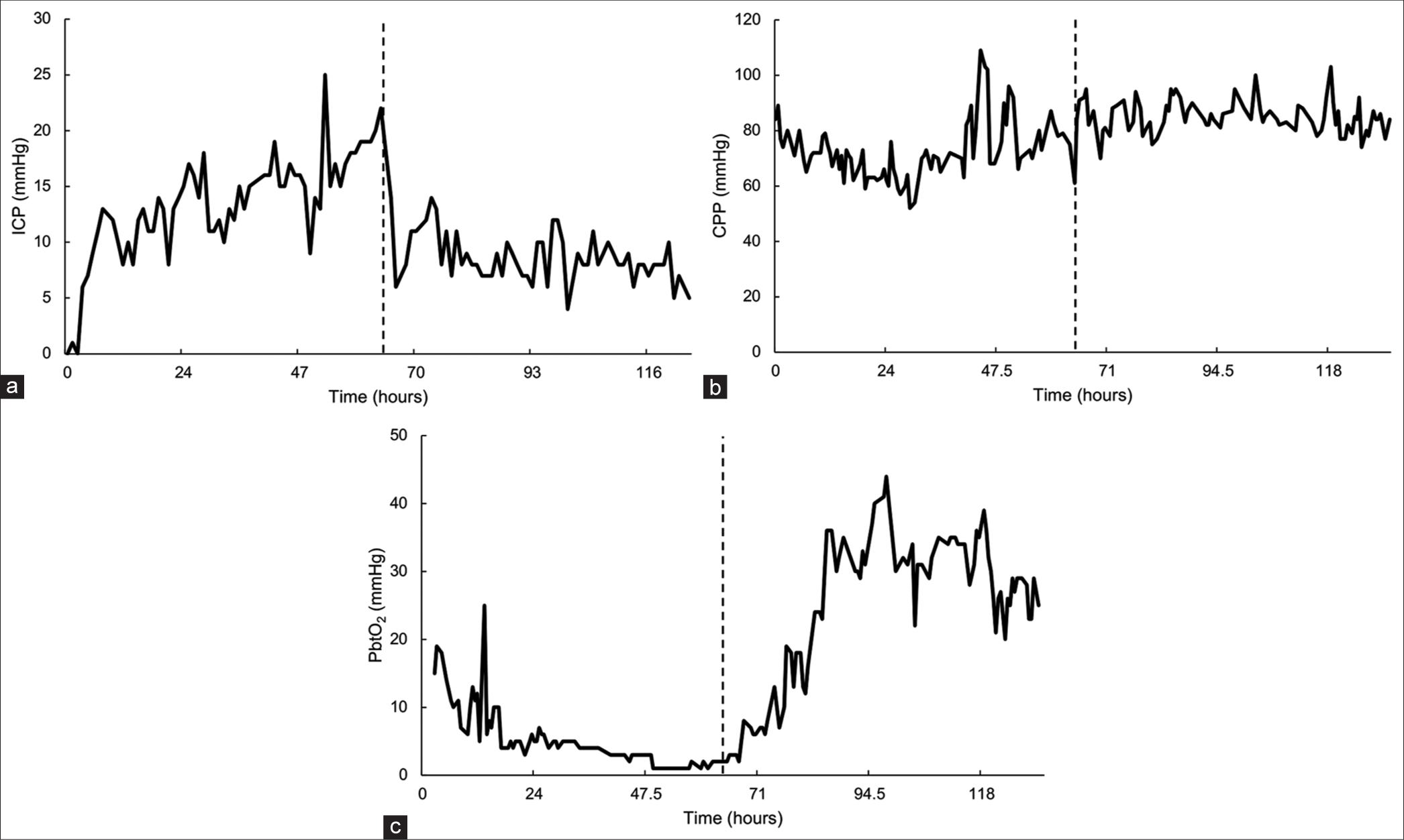
Figure 2:
Neuromonitoring data. (a) Intracranial pressure (ICP) versus time. (b) Cerebral perfusion pressure (CPP) versus time. (c) Brain tissue oxygenation (PbtO2) versus time. All neuromonitors were located in the left frontal lobe. Surgery for intracerebral hemorrhage evacuation occurred at 0 h, and surgical placement of the ventriculostomy drain occurred at 62 h (indicated by the dotted black line).
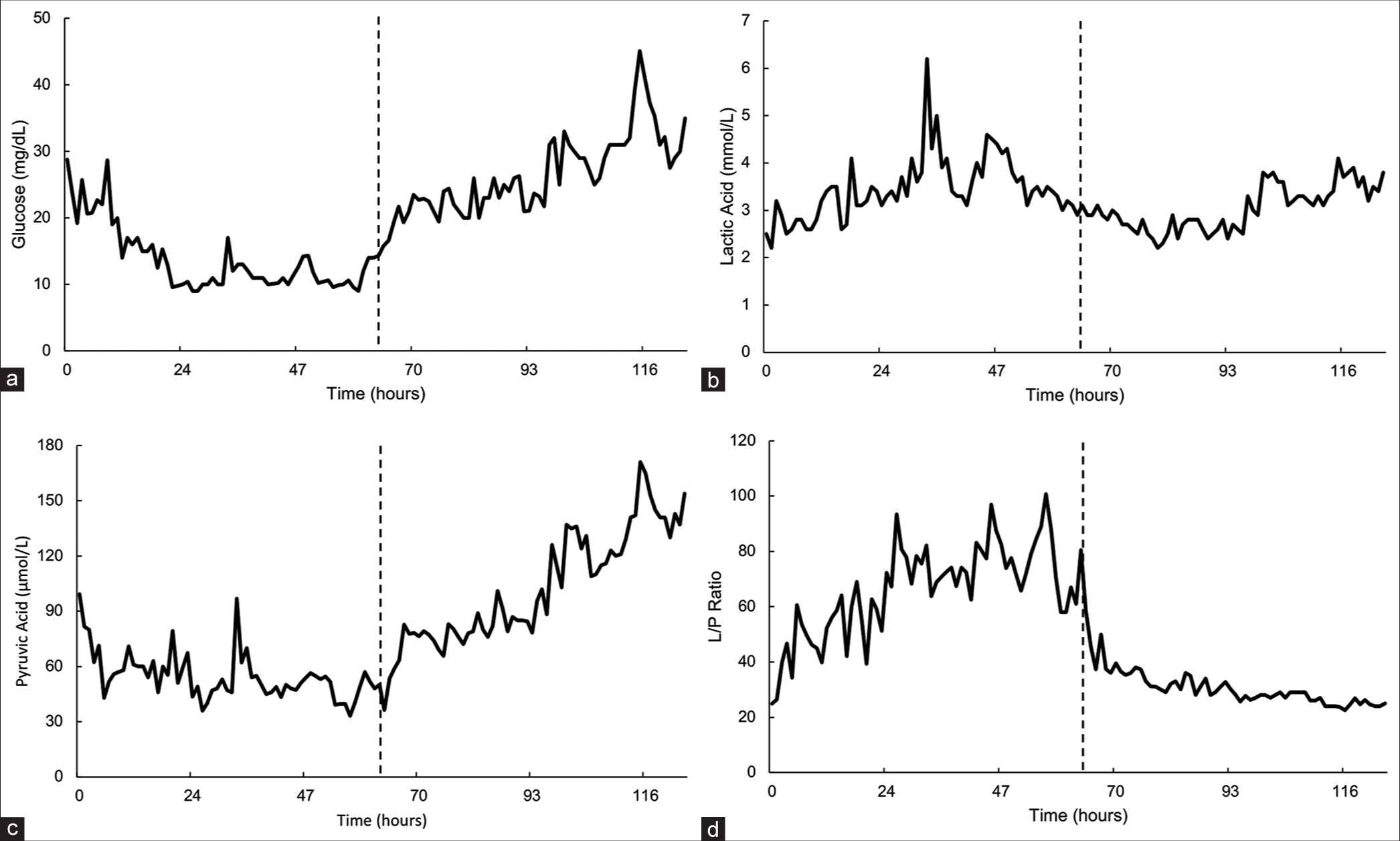
Figure 3:
Cerebral microdialysis data. (a) Glucose versus time. (b) Lactic acid versus time. (c) Pyruvic Acid versus time. (d) Lactate to pyruvate (L/P) ratio versus time collected from cerebral microdialysis catheter in the left frontal lobe. All cerebral microdialysis catheters were located in the left frontal lobe. Surgery for intracerebral hemorrhage evacuation occurred at 0 h, and surgical placement of the ventriculostomy drain occurred at 62 h (indicated by the dotted black line).
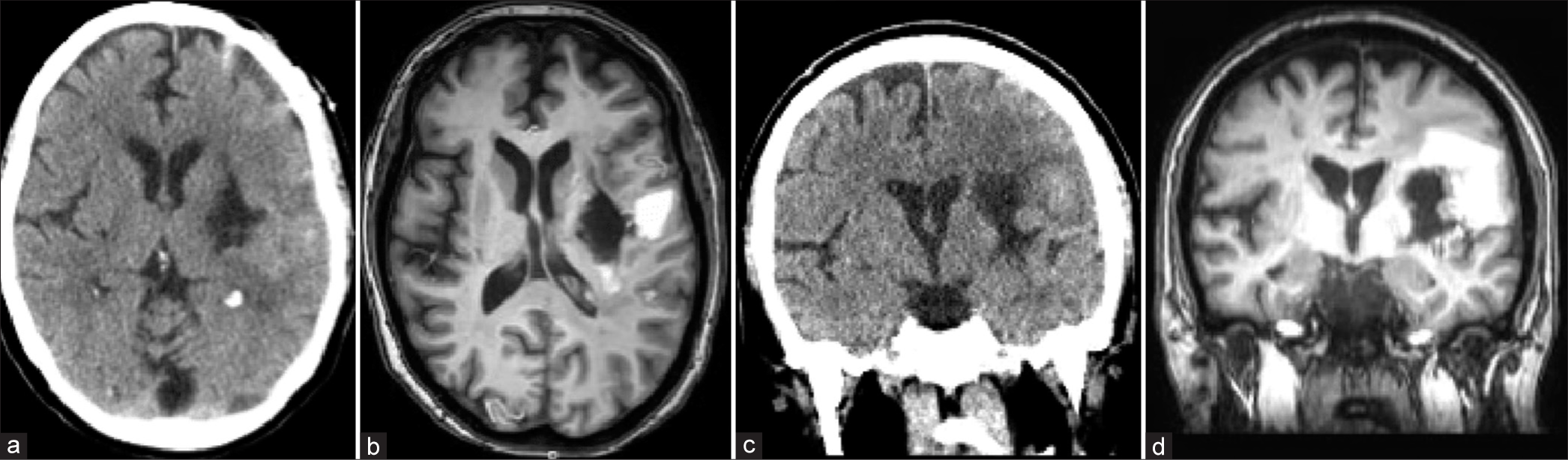
Figure 4:
Computed tomography (CT) and magnetic resonance imaging (MRI) images done three weeks after the initial hemorrhage. (a) Axial CT scan through the area of the hemorrhage. (b) Axial T1 MRI scan through the same area. Note the area of increased signal lateral to the area of hemorrhage evacuation that represents residual hemorrhage that was not removed at the time of the BrainPath™ procedure. (c) Coronal CT scan through the area of the hemorrhage. (d) Coronal T1 MRI scan through the same area. Note the area of residual hemorrhage lateral to the area of hemorrhage. There is an overall decrease in mass effect and surrounding cerebral edema on these images taken about three weeks after the initial hemorrhage. The ventriculostomy has been removed. There is no sign of hydrocephalus.
Operative technique and brain monitor placement
The left basal ganglia hemorrhage was approached through a minimally invasive parafascicular surgery (MIPS) approach with the BrainPath™ Cannula (Nico Corporation, Indianapolis, IN) using a trans sulcal approach along the long access of the blood clot.[
DISCUSSION
In this study, we demonstrate the evacuation of an ICH through MIPS using the BrainPath™ cannula with resultant abnormal multimodal monitor parameters despite adequate resection. Postoperatively, the patient had suboptimal lactate/ pyruvate values, which correlated with low PbtO2, suggesting a metabolic crisis. We demonstrate the first case of clear temporal improvement in metabolic parameters following the placement of EVD in a surgically evacuated sICH.
A striking aspect of this case is the discordance between ICP and metabolic parameters. In the Monroe-Kellie Principle, ICP is inversely affected by increased intracranial volume. High ICPs result in lower cerebral perfusion pressure (CPP) and, therefore, a state of tissue oliguria, subject to whether microcirculatory autoregulation is intact. Explanations for the finding of metabolic crisis despite “normal” ICP include (1) regional lateralized ICP with local CPP compromise in the penumbral zone, (2) peri-hematoma edema increasing local metabolic demand, and (3) disruption of local cerebral autoregulation. This study highlights the utility of metabolic-based multimodal monitoring as a precision medicine device and supports the concept that ICP thresholds may differ between patients.[
Placement of EVD after surgical resection in ICH is up to the discretion of the surgical team and is typically dependent on the degree of hydrocephalus. Recent large randomized trials of minimally invasive ICH evacuations suggest surgery is safe and selected patients possibly have mortality and morbidity benefits.[
Current knowledge regarding using CMD in sICH is limited; however, CMD has more established clinical benefits in SAH and TBI. In a recent microdialysis study, Rasulo et al. observed disturbance of cerebral autoregulation and deranged metabolites in the PHZ for 21 out of 22 patients with sICH.[
The results indicate that using an EVD helped attenuate relatively increasing ICPs and allowed for subsequent improvement in brain oxygenation, cerebral blood flow, and metabolism. Although ICP was in the high-normal range after the initial surgery, further lowering of ICP through EVD placement resulted in improved trends in all recorded metabolic parameters. The results suggest that modification of metabolism in the PHZ, monitored by CMD, can improve outcomes by decreasing the area of potential cell death both temporarily and in the long term. These data highlight CMD’s utility in understanding the underlying metabolic function and as an adjunct to developing treatment plans in patients with sICH. Further studies should investigate the safety and practicality of CMD in multimodal monitoring and its utility in predicting neurologic outcomes in larger prospective cohorts of ICH.
CONCLUSION
This case illustrates the utility of multimodal brain monitoring to improve the local environment of the brain in response to sICH. We also note the removal of the ICH through a minimally invasive approach, followed by the placement of the EVD, which led to improved brain metabolism in the PHZ despite high-normal ICP. When used in this context, CMD may reveal early signs of metabolic dysfunction, indicating the need for further intervention, which may help improve outcomes in sICH
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Carteron L, Bouzat P, Oddo M. Cerebral microdialysis monitoring to improve individualized neurointensive care therapy: An update of recent clinical data. Front Neurol. 2017. 8: 601
2. Carteron L, Solari D, Patet C, Quintard H, Miroz JP, Bloch J. Hypertonic lactate to improve cerebral perfusion and glucose availability after acute brain injury. Crit Care Med. 2018. 46: 1649-55
3. Citerio G, Oddo M, Taccone FS. Recommendations for the use of multimodal monitoring in the neurointensive care unit. Curr Opin Crit Care. 2015. 21: 113-9
4. Cordonnier C, Tymianski M. MISTIE III. Stroke. 2019. 50: 1634-5
5. Foreman B, Ngwenya LB, Stoddard E, Hinzman JM, Andaluz N, Hartings JA. Safety and reliability of bedside, single burr hole technique for intracranial multimodality monitoring in severe traumatic brain injury. Neurocrit Care. 2018. 29: 469-80
6. Güiza F, Depreitere B, Piper I, Citerio G, Chambers I, Jones PA. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015. 41: 1067-76
7. Helbok R, Kofler M, Schiefecker AJ, Gaasch M, Rass V, Pfausler B. Clinical use of cerebral microdialysis in patients with aneurysmal subarachnoid hemorrhage-state of the art. Front Neurol. 2017. 8: 565
8. Kurtz P, Claassen J, Schmidt JM, Helbok R, Hanafy KA, Presciutti M. Reduced brain/serum glucose ratios predict cerebral metabolic distress and mortality after severe brain injury. Neurocrit Care. 2013. 19: 311-9
9. Labib MA, Shah M, Kassam AB, Young R, Zucker L, Maioriello A. The safety and feasibility of image-guided brainpath-mediated transsulcul hematoma evacuation: A multicenter study. Neurosurgery. 2017. 80: 515-24
10. Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ. Brain oxygen optimization in severe traumatic brain injury phase-II: A phase II randomized trial. Crit Care Med. 2017. 45: 1907-14
11. Persson L, Valtysson J, Enblad P, Warme PE, Cesarini K, Lewen A. Neurochemical monitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg. 1996. 84: 606-16
12. Phillips VL, Roy AK, Ratcliff J, Pradilla G. Minimally invasive parafascicular surgery (MIPS) for spontaneous intracerebral hemorrhage compared to medical management: A case series comparison for a single institution. Stroke Res Treat. 2020. 2020: 6503038
13. Rasulo F, Piva S, Park S, Oddo M, Megjhani M, Cardim D. The association between peri-hemorrhagic metabolites and cerebral hemodynamics in comatose patients with spontaneous intracerebral hemorrhage: An international multicenter pilot study analysis. Front Neurol. 2020. 11: 568536
14. Rosenthal G, Sanchez-Mejia RO, Phan N, Hemphill JC, Martin C, Manley GT. Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury. J Neurosurg. 2011. 114: 62-70
15. Sanchez JJ, Bidot CJ, O’Phelan K, Gajavelli S, Yokobori S, Olvey S. Neuromonitoring with microdialysis in severe traumatic brain injury patients. Acta Neurochir Suppl. 2013. 118: 223-7
16. Tobieson L, Gard A, Ruscher K, Marklund N. Intracerebral proinflammatory cytokine increase in surgically evacuated intracerebral hemorrhage: A microdialysis study. Neurocrit Care. 2022. 36: 876-87
17. Tobieson L, Ghafouri B, Zsigmond P, Rossitti S, Hillman J, Marklund N. Dynamic protein changes in the perihaemorrhagic zone of surgically treated intracerebral haemorrhage patients. Sci Rep. 2019. 9: 3181
18. Unterberg AW, Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR. Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2001. 94: 740-9
19. Veldeman M, Albanna W, Weiss M, Park S, Hoellig A, Clusmann H. Invasive multimodal neuromonitoring in aneurysmal subarachnoid hemorrhage: A systematic review. Stroke. 2021. 52: 3624-32
20. Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005. 25: 763-74