- Department of Neurosurgery, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan.
Correspondence Address:
Atsushi Sasahara, Department of Neurosurgery, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan.
DOI:10.25259/SNI_859_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tatsuya Maegawa, Atsushi Sasahara, Hidenori Ohbuchi, Mikhail Chernov, Hidetoshi Kasuya. Cerebral vasospasm and hypoperfusion after traumatic brain injury: Combined CT angiography and CT perfusion imaging study. 19-Jul-2021;12:361
How to cite this URL: Tatsuya Maegawa, Atsushi Sasahara, Hidenori Ohbuchi, Mikhail Chernov, Hidetoshi Kasuya. Cerebral vasospasm and hypoperfusion after traumatic brain injury: Combined CT angiography and CT perfusion imaging study. 19-Jul-2021;12:361. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10977
Abstract
Background: Timely identification of the cerebral perfusion abnormalities after traumatic brain injury (TBI) is highly important. The objective of this study was the evaluation of the post traumatic vasospasm and cerebral hypoperfusion with the serial combined CT angiography (CTA) and CT perfusion (CTP) imaging examinations.
Methods: The case series comprised 25 adult patients with closed TBI accompanied by various types of intracranial hematoma. Emergency surgery was done in 15 cases (60%). Combined CTA and CTP were performed on days 0 (D0) and 7 ± 1 (D7) after trauma.
Results: CTA on D0 did not demonstrate vasospasm in any case but revealed it on D7 in 9 patients (36%). In the multivariate analysis, only the presence of subarachnoid hemorrhage (SAH) on D7 had confirmed a significant association with the development of vasospasm (P = 0.0201). Cerebral hypoperfusion at least in one evaluated brain region was noted on D0 and D7 in 76% and 60% of patients, respectively, and showed highly variable spatial distribution and temporal development. Treatment results were not associated with the presence of vasospasm (P = 0.7337) or the number of brain regions affected by hypoperfusion on D0 (P = 0.2285), but the number of brain regions affected by hypoperfusion on D7 was significantly greater in cases of unfavorable outcome (P = 0.0187).
Conclusion: Vasospasm is merely related to SAH sustained at the subacute stage of TBI, but its spatial and temporary interrelationships with the post traumatic cerebral hypoperfusion are complex. Serial combined CTA and CTP examinations may facilitate monitoring of perfusion abnormalities and treatment guidance.
Keywords: Cerebral hypoperfusion, CT angiography, CT perfusion imaging, Post traumatic vasospasm, Traumatic brain injury
INTRODUCTION
Management of acute severe traumatic brain injury (TBI) still represents a significant challenge. Contemporary treatment protocols are mainly directed at the preservation of the intracranial pressure (ICP) and cerebral perfusion pressure within the normal range using a variety of means, both therapeutic and surgical, and are strongly influenced by the imaging data, usually obtained with plain head CT. However, this modality can provide only structural information and does not assess the physiological alterations of cerebral blood flow (CBF), while their timely identification may be rather important. In fact, severe perfusion abnormalities after TBI are rather common (2–9% of cases) and may result in a significant increase in morbidity and mortality.[
According to the standard protocol adopted in the Tokyo Women’s Medical University Medical Center East, all patients with TBI routinely undergo plain head CT at admission, and if intracranial hemorrhage is demonstrated, CT angiography (CTA) and CT perfusion (CTP) imaging are additionally performed at the same setting to facilitate differential diagnosis with the cerebrovascular accident (CVA). These radiological data were utilized for the present retrospective study directed at the evaluation of the post traumatic cerebral vasospasm and hypoperfusion.
MATERIALS AND METHODS
This case series comprised 25 consecutive adult patients with closed TBI accompanied by the various types of intracranial hematoma, who were admitted to our center from January 2012 to September 2015 and were evaluated by means of CTA and CTP both at acute and subacute stages, that is, on days 0 (D0) and 7 ± 1 (D7) after trauma. Informed consent before these examinations and any invasive intervention (including surgery) was provided by each patient and/or his/her nearest family member. CTA and CTP investigations at admission were not considered in moribund patients with fixed dilated pupils, patients with TBI combined with other injuries, as well as in cases of renal failure or allergy for iodine-based contrast medium.
The final analysis of data was done in May 2020 and was independently performed by two authors (T.M.; A.S.) with resolution of all disagreements on discussion. All evaluated variables were extracted from the electronic medical records. No one case was excluded from the study cohort. Research protocol was approved by the Ethics Committee of Tokyo Women’s Medical University (No. 2883).
Treatment
All patients were treated in the dedicated Neuro-ICU, and 15 of them (60%) underwent emergency surgery directed at the evacuation of the intracranial hematomas and/or external decompression in the presence of severe brain swelling. In all cases of severe TBI intraparenchymal sensor for prolonged ICP monitoring were inserted. Treatment with barbiturate-induced coma was applied if deemed necessary, but therapeutic hypothermia was not utilized. In general, treatment strategy was not modified according to results of CTA and CTP examinations, both of which on D0 were consistently performed before any surgical intervention.
CTA and CTP imaging
CTA and CTP investigations were done by means of 64-detector row CT scanners (LightSpeed VCT XT or Discovery CT750 HD; GE Healthcare, Milwaukee, WI, USA) with the use of toggling-table technique (VolumeShuttle; GE Healthcare) to extend the coverage to 80 mm in the Z-axis for the perfusion scan with the following parameters: tube voltage, 80 kVp; tube current, 180 mA; rotation time, 0.4 s; sampling interval, 2.8 s. The intravenous injection of 40 mL of iodine-based contrast medium (Iopamiron 370; Bayer Schering Pharma AG, Berlin-Wedding, Germany) was followed by a 20 mL saline flush. To reduce image noise artifacts and to achieve a low-volume CT dose index of 85 mGy during the multiphase CTP scanning, an adapted statistical iterative reconstruction algorithm (GE Healthcare) for perfusion source images was used.
The post processing transformation of the acquired data into CBF, cerebral blood volume, and mean transit time (MTT) maps was performed within the Advantage Workstation (GE Healthcare) using CTP software, Version 3.0 (GE Healthcare), which is based on the deconvolution method and generally considered as highly accurate technique for the perfusion studies with low contrast injection rate. The technique was standardized for all patients according to the recommended guidelines with arterial input and venous output functions measured, respectively, at the A2 segment of the anterior cerebral artery (ACA) and the superior sagittal sinus.
Analysis of CTA and CTP data was done by two neurosurgeons highly experienced with these imaging modalities (T.M.; A.S.), who were blinded to the clinical details of evaluated cases. The presence of vasospasm was considered if CTA demonstrated segmental or diffuse narrowing of more than 50% of the blood vessel diameter in large cerebral arteries. Hypoperfusion on CTP was assessed qualitatively based on the determination of MTT prolongation in brain regions, corresponding to the major vascular territories of ACA, middle cerebral arteries (MCA), and posterior cerebral arteries (PCA) on both sides (i.e., in six separate brain regions per each case).
Outcome assessment
Outcome was assessed at the time of discharge from the hospital according to the Glasgow Outcome Scale (GOS) and was considered as favorable (GOS scores 4–5) or unfavorable (GOS scores 1–3).
Statistical analysis
The following factors were compared between different subgroups of patients: gender (men vs. women), age (<52 years vs. >52 years), cause of TBI (yes vs. no for each individual cause), Glasgow Coma Scale (GCS) score at admission (9–15 vs. 3–8), imaging findings on plain head CT on D0 and D7 (yes vs. no for each individual finding), treatment (surgery vs. pure conservative therapy with or without ICP monitoring), and outcome (favorable vs. unfavorable). Comparison of numerical and categorical variables was done, respectively, with the Student’s t-test and Chi-square test. Factors, which showed statistically significant associations with the variable of interest in the univariate analysis, were included in the multivariate model. For all calculations, the commercially available software JMP® Pro 15 (SAS Institute Inc.; Cary, NC) was used. The level of statistical significance was defined at P < 0.05.
RESULTS
The clinical characteristics of the case series are presented in [
Post traumatic vasospasm
CTA on D0 did not demonstrate post traumatic vasospasm in any case but showed it on D7 in nine patients (36%); their characteristics are presented in [
In comparison to patients without post traumatic vasospasm, those with vasospasm significantly more often had subdural (89% vs. 44%; P = 0.0270) and intraventricular (22% vs. 0%; P = 0.0493) hematomas on D0 (i.e., at admission) and SAH on D7 (89% vs. 31%; P = 0.0056), and less frequent presence of intracerebral hemorrhage on D7 (0% vs. 44%; P = 0.0194). Other evaluated factors did not show statistically significant difference between subgroups of patients with and without post traumatic vasospasm. However, in the multivariate model based on logistic regression, only the presence of SAH on D7 has confirmed its independent statistically significant association with the development of post traumatic vasospasm (P = 0.0201). Identification of SAH on D7 predicted the presence of post traumatic vasospasm with 0.89 sensitivity, 0.69 specificity, 0.62 positive predictive value (PPV), 0.92 negative predictive value (NPV), and 0.76 accuracy.
Cerebral hypoperfusion
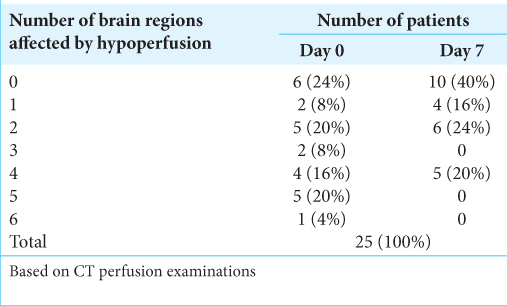
According to CTP, the number of brain regions affected by hypoperfusion on D0 and D7 varied, respectively, from 0 to 6/ patient (mean, 2.6/patient) and from 0 to 4/patient (mean, 1.4/ patient); the details of their distribution are shown in [
Overall, cerebral hypoperfusion on D0 was identified in 65 (43%) out of 150 evaluated brain regions. In comparison to their counterparts, the mean number of brain regions affected by hypoperfusion on D0 was significantly greater in patients with GCS score 3–8 at admission (3.9 vs. 2.1; P = 0.0475), those who had subdural hematoma (3.5 vs. 1.3; P = 0.0051) and SAH (3.1 vs. 1.3; P = 0.0383) on D0 (i.e., at admission), those who underwent surgical treatment (3.3 vs. 1.5; P = 0.0214), and those who had intracerebral hemorrhage on D7 (4.0 vs. 2.1; P = 0.0257). Other evaluated factors did not show statistically significant associations with the number of brain regions affected by hypoperfusion on D0. However, in the multivariate model based on multiple regression, none of the aforementioned factors has confirmed its independent statistically significant association with the number of brain regions affected by hypoperfusion on D0.
Overall, cerebral hypoperfusion on D7 was identified in 36 (24%) out of 150 evaluated brain regions. In comparison to their counterparts, the mean number of brain regions affected by hypoperfusion on D7 was significantly greater in patients with GCS score 3–8 at admission (2.4 vs. 1.1; P = 0.0416), those who had subdural hematoma (2.1 vs. 0.4; P = 0.0032) and SAH (1.8 vs. 0.4; P = 0.0367) on D0 (i.e., at admission), and those who had brain contusion (2.1 vs. 0.6; P = 0.0166), intracerebral hemorrhage (2.9 vs. 0.9; P = 0.0019), and SAH (2.2 vs. 0.7; P = 0.0123) on D7. Other evaluated factors did not show statistically significant associations with the number of brain regions affected by hypoperfusion on D7. However, in the multivariate model based on multiple regression, only GCS score 3–8 at admission (P = 0.0107) and presence of intracerebral hemorrhage on D7 (P = 0.0255) have confirmed their independent statistically significant association with the greater number of brain regions affected by hypoperfusion on D7.
The number of brain regions affected by hypoperfusion on D0 and D7 was significantly associated with each other (P = 0.0003). Identification of the cerebral hypoperfusion on D0 predicted its presence within the same brain region on D7 with 0.69 sensitivity, 0.65 specificity, 0.38 PPV, 0.87 NPV, and 0.66 accuracy.
Interrelationships of post traumatic vasospasm and cerebral hypoperfusion
In nine patients with post traumatic vasospasm, cerebral hypoperfusion on D0 and D7 was revealed, respectively, in 32 (59%) and 22 (41%) of 54 evaluated brain regions. In this subgroup, in 17 brain regions (31%) hypoperfusion was marked on both D0 and D7, in 15 (28%) on D0 only, in 5 (9%) on D7 only, and in 17 (31%) neither on D0 nor on D7. The mean number of brain regions affected by hypoperfusion on D0 did not differ significantly between subgroups of patients with and without post traumatic vasospasm (3.6 vs. 2.1/ patient; P = 0.0687). In contrast, the mean number of brain regions affected by hypoperfusion on D7 was significantly greater in the subgroup of patients with post traumatic vasospasm (2.4 vs. 0.9/patient; P = 0.0106).
Out of 16 vascular territories affected by the post traumatic vasospasm on D7, the cerebral hypoperfusion in corresponding brain regions was noted on D0 and D7 in 12 (75%) and 11 (69%) of cases, respectively. Identification of the cerebral hypoperfusion on D0 predicted the development of post traumatic vasospasm in corresponding vascular territory on D7 with 0.75 sensitivity, 0.60 specificity, 0.18 PPV, 0.95 NPV, and 0.62 accuracy. Identification of the cerebral hypoperfusion on D7 predicted the presence of post traumatic vasospasm in the corresponding vascular territory with 0.69 sensitivity, 0.81 specificity, 0.31 PPV, 0.96 NPV, and 0.80 accuracy.
Outcome
Overall, favorable and unfavorable outcomes were noted in 15 (60%) and 10 (40%) cases, respectively. The number of unfavorable outcomes did not differ significantly between subgroups of patients with and without post traumatic vasospasm (44% vs. 38%; P = 0.7337). Furthermore, the outcome was not significantly associated with the number of brain regions affected by hypoperfusion on D0 (mean, 2.2 vs. 3.2/patient in cases of favorable and unfavorable outcome, respectively; P = 0.2285). In contrast, the number of brain regions affected by hypoperfusion on D7 was significantly greater in cases of unfavorable outcome (mean, 0.9 vs. 2.3/ patient in cases of favorable and unfavorable outcome, respectively; P = 0.0187).
Illustrative case
A 77-year-old woman fell from the staircase and was transferred to our center by ambulance. At admission, her GCS score was 9 (eye opening, 2; verbal response, 2; motor response, 5). Plain head CT revealed acute subdural hematoma above the right cerebral convexity causing brain shift, as well as multiple brain contusions, intracerebral hemorrhages, and SAH, whereas CTA defined spot signs within the anterior right temporal and posterior left temporal lobes [
Figure 1:
Neuroimaging findings in a 77-year-old woman with traumatic brain injury. Plain head CT at admission (a) revealed acute subdural hematoma above the right cerebral convexity causing brain shift, as well as multiple brain contusions, intracerebral hemorrhages, and subarachnoid hemorrhage, whereas CT angiography (CTA) (b) defined spot signs (arrows) within the anterior right temporal and posterior left temporal lobes. One hour later prominent expansion of the intracerebral hemorrhages was noted (c), thus urgent right-sided decompressive craniectomy and the evacuation of subdural hematoma were done. On the 8th day after trauma and surgery, CTA (d and e) revealed localized vasospasm of the left M2 segment (arrows), whereas CT perfusion (f) showed marked hypoperfusion of the left frontal and temporal lobes (circle). Subsequent diffusion-weighted imaging (g and h) demonstrated multiple ischemic lesions (arrows) in both hemispheres.
DISCUSSION
The current concept of TBI considers brain contusion, intracerebral hemorrhages, intracranial hematomas, corresponding abnormalities of CBF, acute disruption of the blood-brain barrier, and brain edema as primary brain injury caused by trauma itself, whereas the following secondary brain injury is mainly related to the cerebral hypoperfusion, alterations of the metabolite transport, and neuronal tissue disorders.[
Extensive research has been done in the past on post traumatic vasospasm, which affects 27–40% of patients with TBI.[
On the other hand, the results presented herein also demonstrated that in 75% of cases the post traumatic vasospasm revealed by CTA on D7 has appeared in the brain regions affected by the cerebral hypoperfusion on D0. Such an association can be explained considering the concept of neurovascular unit (NVU), which presumes the presence of the cell-level component of the cerebrovascular system, consisting of neurons, astrocytes, endothelial cells, pericytes, and smooth muscle cells. The brain injury resulting in neurovascular tissue damage may cause degeneration of the cellular components of NVU and its functional dysregulation with possible excess of endothelin-1, a vasoconstrictor secreted by pericytes and closely related to the development of post traumatic vasospasm.[
Complex spatial and temporary interrelationships of the vasospasm with cerebral hypoperfusion probably reflect the comprehensive interplay of multiple related factors and underlying mechanisms. Based on the findings of the presented study it can be speculated that pathophysiological reactions leading to the development of vasospasm after TBI are primarily induced by the early post traumatic ischemic brain tissue damage, and further augmented by the prolonged presence of blood products in the subarachnoid cisterns.
Cerebral hypoperfusion at least in one evaluated brain region was noted on D0 and D7 in 76% and 60% of our patients, respectively. Moreover, the outcome in the analyzed cohort was significantly related to the number of brain regions affected by hypoperfusion at the subacute stage of TBI (i.e., on D7), which, in turn, was related to GCS score at admission and presence of intracerebral hemorrhage on D7. Nevertheless, post traumatic cerebral hypoperfusion has demonstrated highly variable spatial distribution and temporal development. In some cases, it did not show significant changes between CTP examinations on D0 and D7, in others it regressed with time, or, contrary, appeared de novo or was augmented affecting additional brain regions. Such dynamic changes can be hardly predictable based on clinical data or structural imaging. For instance, multivariate analysis did not reveal any factor demonstrating a statistically significant association with the presence of cerebral hypoperfusion at admission (i.e., on D0), which by itself has limited value for prediction of cerebral hypoperfusion at the subacute stage of TBI (i.e., on D7) with PPV as low as 0.38. Thus, to facilitate timely delivery of the appropriate treatment, monitoring of perfusion abnormalities with specific examinations seems necessary.[
Importance of CTA and CTP examinations after TBI
Transcranial Doppler (TCD) is widely used in the neurosurgical practice for the evaluation of cerebral vasospasm. This method is rather convenient, since does not require administration of any contrast media, allows bedside investigation without the need for in-hospital patient transfers, and easily repeatable. On the other hand, TCD examination is time-consuming and demands sufficient expertise of the operator. Moreover, some reports indicated difficulties in TCD assessment of vasospasm in the ACA and PCA,[
The variety of methods for clinical evaluation of the cerebral perfusion includes positron emission tomography, single photon emission computed tomography, and xenon-enhanced CT, but since their development in the late 1990s, the contrast-enhanced CTA and CTP got the widest acceptance in practical neurosurgery due to such advantages as simplicity, speed of investigation, low operator dependence, and unnecessary cooperation of the patient.[
Although in our previous study on high-grade aSAH quantitative evaluation of the CTP data was done,[
Study limitations
The main limitations of the presented study are related to its single-center basis, retrospective design, a small number of investigated cases, and evaluation of the outcome only at the time of discharge from the hospital. Medical history of patients was not available for detailed assessment, thus information on risk factors for CVA, such as arterial hypertension, diabetes mellitus, dyslipidemia, smoking, and alcohol abuse,[
CONCLUSION
In the present study, post traumatic vasospasm affected one third of patients and its presence was merely related to SAH sustained at the subacute stage of TBI. Cerebral hypoperfusion at least in one evaluated brain region was noted at admission and subacute stage of TBI in, respectively, 76% and 60% of cases, and showed highly variable spatial distribution and temporal development. According to our results, the outcome after TBI is strongly associated with the number of brain regions affected by hypoperfusion at the subacute stage of TBI (i.e. on D7). It emphasizes the importance of aggressive therapy preventing development and progression of the cerebral perfusion abnormalities. Such treatment may be significantly facilitated and effectively guided by the serial combined CTA and CTP examinations.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Mufti F, Amuluru K, Changa A, Lander M, Patel N, Wajswol E. Traumatic brain injury and intracranial hemorrhage-induced cerebral vasospasm: A systematic review. Neurosurg Focus. 2017. 43: E14
2. Al-Mufti F, Amuluru K, Lander M, Mathew M, El-Ghanem M, Nuoman R. Low Glasgow coma score in traumatic intracranial hemorrhage predicts development of cerebral vasospasm. World Neurosurg. 2018. 120: e68-71
3. Alves JE, Carneiro  Xavier J. Reliability of CT perfusion in the evaluation of the ischaemic penumbra. Neuroradiol J. 2014. 27: 91-5
4. Aminmansour B, Ghorbani A, Sharifi D, Shemshaki H, Ahmadi A. Cerebral vasospasm following traumatic subarachnoid hemorrhage. J Res Med Sci. 2009. 14: 343-8
5. Bendinelli C, Bivard A, Nebauer S, Parsons MW, Balogh ZJ. Brain CT perfusion provides additional useful information in severe traumatic brain injury. Injury. 2013. 44: 1208-12
6. Bendinelli C, Cooper S, Evans T, Bivard A, Pacey D, Parson M. Perfusion abnormalities are frequently detected by early CT perfusion and predict unfavourable outcome following severe traumatic brain injury. World J Surg. 2017. 41: 2512-20
7. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980. 6: 1-9
8. Greenberg ED, Gold R, Reichman M, John M, Ivanidze J, Edwards AM. Diagnostic accuracy of CT angiography and CT perfusion for cerebral vasospasm: A meta-analysis. AJNR Am J Neuroradiol. 2010. 31: 1853-60
9. Hankey GJ. Stroke. Lancet. 2017. 389: 641-54
10. Honda M, Ichibayashi R, Yokomuro H, Yoshihara K, Masuda H, Haga D. Early cerebral circulation disturbance in patients suffering from severe traumatic brain injury (TBI): A xenon CT and perfusion CT study. Neurol Med Chir (Tokyo). 2016. 56: 501-9
11. Huang AP, Tsai JC, Kuo LT, Lee CW, Lai HS, Tsai LK. Clinical application of perfusion computed tomography in neurosurgery. J Neurosurg. 2014. 120: 473-88
12. Jullienne A, Obenaus A, Ichkova A, Savona-Baron C, Pearce WJ, Badaut J. Chronic cerebrovascular dysfunction after traumatic brain injury. J Neurosci Res. 2016. 94: 609-22
13. Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C. Cerebral vascular injury in traumatic brain injury. Exp Neurol. 2016. 275: 353-66
14. Kobata H. Diagnosis and treatment of traumatic cerebrovascular injury: Pitfalls in the management of neurotrauma. Neurol Med Chir (Tokyo). 2017. 57: 410-7
15. Kowalski RG, Haarbauer-Krupa JK, Bell JM, Corrigan JD, Hammond FM, Torbey MT. Acute ischemic stroke after moderate to severe traumatic brain injury: Incidence and impact on outcome. Stroke. 2017. 48: 1802-9
16. Malinova V, Dolatowski K, Schramm P, Moerer O, Rohde V, Mielke D. Early whole-brain CT perfusion for detection of patients at risk for delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg. 2016. 125: 128-36
17. Oertel M, Boscardin WJ, Obrist WD, Glenn TC, McArthur DL, Gravori T. Posttraumatic vasospasm: The epidemiology, severity, and time course of an underestimated phenomenon: A prospective study performed in 299 patients. J Neurosurg. 2005. 103: 812-24
18. Salehi A, Zhang JH, Obenaus A. Response of the cerebral vasculature following traumatic brain injury. J Cereb Blood Flow Metab. 2017. 37: 2320-39
19. Samagh N, Bhagat H, Jangra K. Monitoring cerebral vasospasm: How much can we rely on transcranial Doppler. J Anaesthesiol Clin Pharmacol. 2019. 35: 12-8
20. Sanelli PC, Nicola G, Johnson R, Tsiouris AJ, Ougorets I, Knight C. Effect of training and experience on qualitative and quantitative CT perfusion data. AJNR Am J Neuroradiol. 2007. 28: 428-32
21. Sasahara A, Suzuki K, Takahashi Y, Koseki H, Hirota K, Ohbuchi H. Prognostic assessment of aneurysmal subarachnoid patients with WFNS grade V by CT perfusion on arrival. World Neurosurg. 2016. 92: 1-6
22. Shahlaie K, Keachie K, Hutchins IM, Rudisill N, Madden LK, Smith KA. Risk factors for posttraumatic vasospasm. J Neurosurg. 2011. 115: 602-11
23. Takahashi Y, Sasahara A, Yamazaki K, Inazuka M, Kasuya H. Disturbance of CT perfusion within 24 h after onset is associated with WFNS grade but not development of DCI in patients with aneurysmal SAH. Acta Neurochir (Wien). 2017. 159: 2319-24
24. van der Harst JJ, Luijckx GR, Elting JW, Bokkers RP, van den Bergh WM, Eshghi OS. Transcranial Doppler versus CT-angiography for detection of cerebral vasospasm in relation to delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: A prospective single-center cohort study; the transcranial Doppler and CT-angiography for investigating cerebral vasospasm in subarachnoid hemorrhage (TACTICS) study. Crit Care Explor. 2019. 1: e0001
25. Wintermark M, van Melle G, Schnyder P, Revelly JP, Porchet F, Regli L. Admission perfusion CT: Prognostic value in patients with severe head trauma. Radiology. 2004. 232: 211-20