- Departments of Neurosurgery, Head and Neck Surgery, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-Ku, Tokyo, Japan.
- Departments of Otorhinolaryngology, Head and Neck Surgery, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-Ku, Tokyo, Japan.
Correspondence Address:
Hiroki Yamada
Departments of Neurosurgery, Head and Neck Surgery, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-Ku, Tokyo, Japan.
DOI:10.25259/SNI_117_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Yamada, Masahiro Toda, Mariko Fukumura, Ryotaro Imai, Hiroyuki Ozawa, Kaoru Ogawa, Kazunari Yoshida. Cerebrospinal fluid leakage due to nasoseptal flap partial necrosis: A pitfall for skull base reconstruction of endoscopic endonasal surgery. 23-May-2020;11:121
How to cite this URL: Hiroki Yamada, Masahiro Toda, Mariko Fukumura, Ryotaro Imai, Hiroyuki Ozawa, Kaoru Ogawa, Kazunari Yoshida. Cerebrospinal fluid leakage due to nasoseptal flap partial necrosis: A pitfall for skull base reconstruction of endoscopic endonasal surgery. 23-May-2020;11:121. Available from: https://surgicalneurologyint.com/surgicalint-articles/10039/
Abstract
Background: Vascularized nasoseptal flaps allow for the reconstruction of large dural defects and have remarkably reduced the incidence of postoperative complications during endoscopic endonasal skull base surgery. Nevertheless, some complications related to nasoseptal flap have been reported. Flap necrosis is a rare, but serious issue is associated with meningitis and cerebrospinal fluid (CSF) leak.
Case Description: We performed endoscopic endonasal removal of the tuberculum sella meningioma in a 39-year-old woman with a history of Turner syndrome using abdominal fat, fascia, and a vascularized nasoseptal flap for dural and skull base defect reconstruction. After surgery, she developed CSF leak, and reoperation revealed partial necrosis of the septal flap that caused leakage. At this time, with a concern that removal of the necrotic part may lead to the insufficient size of the flap, we filled the gap tightly with fat pieces. However, the CSF leak recurred, and thus, we performed debridement of the necrotic region and reformed the multilayered reconstruction, following which she no longer experienced CSF leakage.
Conclusion: Our case suggested that partial rather than total flap necrosis could occur, possibly due to variances of vascular anatomy, leading to focal ischemia. Debridement of the necrotic region may be an important solution for recurrent cerebrospinal leakage secondary to partial necrosis of a nasoseptal flap.
Keywords: Cerebrospinal fluid leak, Meningioma, Nasoseptal flap, Necrosis, Skull base
INTRODUCTION
Advances in endoscopic endonasal surgery (EES) have expanded its indications to skull base lesions and have enabled safer and less invasive surgery. Cerebrospinal fluid (CSF) leakage is a complication that requires the most attention when performing skull base lesions by EES, and it primarily occurred at a considerably high rate. As a pivotal solution, vascularized nasoseptal flap (NSF), which was introduced by Hadad et al.,[
Recently, complications related to NSF have been studied. In a systematic review, Lavine et al. reported that the complications of NSF were mucocele formation, septal perforation, nasal dorsum collapse, effects on quality of life, olfactory loss, and flap necrosis.[
We report a case of NSF partial necrosis caused recurrent CSF leakage and subsequent meningitis. We discuss possible causes and surgical pitfalls associated with its repair.
CASE DESCRIPTION
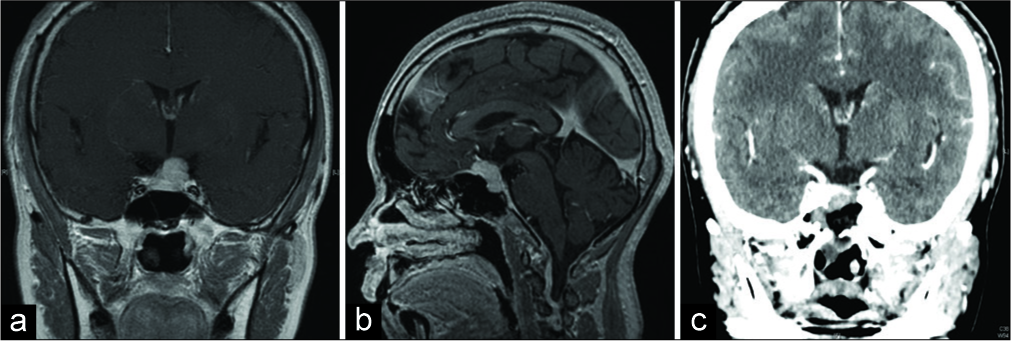
A 39-year-old woman with a clinical history of Turner syndrome (treated with estrogen and progesterone), hypercholesterolemia, and chronic thyroiditis, complained that vision in her left eye had turned whitish and hazy during desk work. Examination revealed that her visual acuity of the left eye had decreased to 20/100 vision (right eye was 20/20 vision) with a superior visual field defect of her left eye. Magnetic resonance imaging revealed a 19 mm suprasellar and sellar mass lesion with contrast enhancement [
We performed endoscopic endonasal removal of the tumor with a team including neurosurgeons and head-and-neck surgeons. Initially, a pedunculated full NSF was created by approaching from the right nasal cavity as our routine; with an awareness of preserving the posterior septal branch of the sphenopalatine artery, a vertical incision was initiated at the level of the sphenoid ostium and extended anteriorly until just posterior of the mucosa-skin transition. The gross total removal (Simpson Grade 2) of the tumor was achieved. For skull base defect, multilayer reconstruction was performed by inlaying the fascia collected from the abdomen, cloaking the NSF on to the dural defect, and then onlaying fat collected from the abdomen in front of it. The histopathological diagnosis of the tumor was angiomatous meningioma.
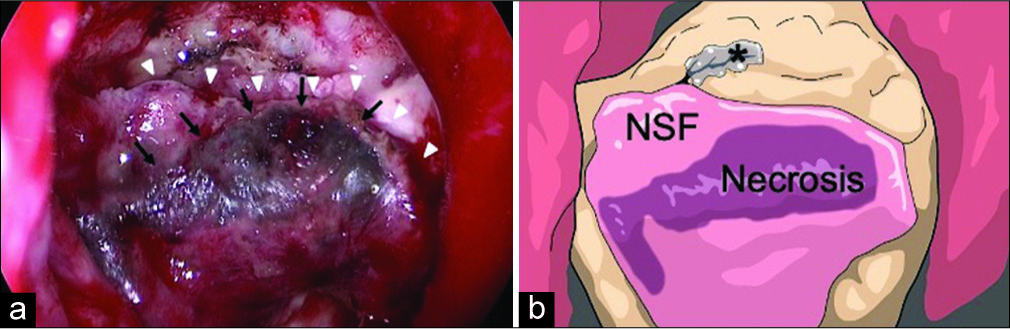
After surgery, she experienced transient deterioration of the left visual acuity; therefore, steroid pulse therapy was administered 3 times. The visual acuity slowly recovered to preoperative level. She had steadily regained ambulation. However, on the 7th postoperative day (POD), she developed clear fluid dripping from the right nostril, which was suspected to represent CSF leakage. Conservative treatment with bed rest did not improve the symptom, so we performed EES again. During surgery, careful removal of the packing around the planum sphenoidale and sella tunica revealed that the superior half of the NSF was partially necrotic and shifted posteriorly [
Figure 2:
(a) Intrasurgical view at the first reoperation showing partial necrosis (black arrows) of the nasoseptal flap (NSF) (white arrowheads) and cerebrospinal fluid (CSF) leakage from the above. (b) Schematic drawing of Figure 2a which clearly shows the NSF, flap necrosis, and CSF leak point (asterisk).
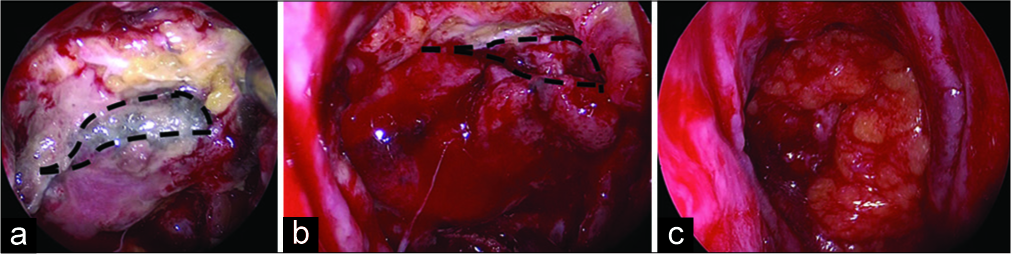
Postoperatively, she gradually regained ambulation; however, CSF leak appeared again 6 days after the reoperation. She also developed fever, headache, and increased serum inflammatory responses suggestive of meningitis. We again performed emergent EES [
Figure 3:
Intrasurgical view at the second reoperation revealing (a) partial necrosis of the nasoseptal flap and fat pieces that were prevented from engrafting; the area within the dashed line represents the area planned for debridement; (b) after debridement of the necrotic region, the area within the dashed line represents the actual debrided region; (c) multi-reconstruction of the dural defect done with the fascia, non-necrotic flap, and fat pieces.
DISCUSSION
Vascularized NSF has been reliable and is the most- used method today for anterior and ventral skull base reconstruction during EES. It is reported that it has reduced the incidence of CSF leakage from 15.6% to 6.7%.[
Various factors can be considered as risk factors for flap necrosis: patient characteristics, intracranial hypertension, flap rotation, pedicle compression, infection, and radiation therapy.[
If a flap partial necrosis caused CSF leak, it may insufficient simply to fill the gap. This is because the necrosis discourages engrafting the filling material such as fat and fascia well. In our case, at first reoperation, instead of debridement, we closed the gap with the fat onlay. This was due to concerns that debriding would reduce the size of the flap, making it impossible to cover the large dural defect at the base of the skull, and resulting in insufficient reconstruction. The gross cessation of CSF leakage and the findings of the Valsalva procedure seemed appropriate at this point. However, the fat did not firmly fix because there was no blood flow from the flap. Therefore, we believe that the pitfall in this case was the necessity of debridement of the necrotic region. In fact, past reports of septal necrosis describe aggressive debridement and redone multilayer reconstruction.[
Turner syndrome patients have been reported to be at an increased risk for meningioma.[
CONCLUSION
We treated a rare case of EES complicated with recurrent CSF leakage because of NSF partial necrosis. Our case suggests that the partial, not entire, flap necrosis could occur. The cause may be an anatomical variance that led to local ischemia and steroid treatment may also have played a role. As a solution, the debridement of the necrotic region may be necessary.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amelot A, Lemaistre G, Cornu P, Kalamarides M, Peyre M. Multiple meningiomas in patients with Turner syndrome. Acta Neurochir (Wien). 2015. 157: 621-3
2. Chabot JD, Patel CR, Hughes MA, Wang EW, Snyderman CH, Gardner PA. Nasoseptal flap necrosis: A rare complication of endoscopic endonasal surgery. J Neurosurg. 2018. 128: 1463-72
3. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006. 116: 1882-6
4. Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscopic skull base reconstruction of large dural defects: A systematic review of published evidence. Laryngoscope. 2012. 122: 452-9
5. Lavigne P, Faden DL, Wang EW, Snyderman CH. Complications of nasoseptal flap reconstruction: A systematic review. J Neurol Surg B Skull Base. 2018. 79: S291-9
6. Pier DB, Nunes FP, Plotkin SR, Stemmer-Rachamimov AO, Kim JC, Shih HA. Turner syndrome and meningioma: Support for a possible increased risk of neoplasia in Turner syndrome. Eur J Med Genet. 2014. 57: 269-74
7. Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA. Cancer incidence in women with Turner syndrome in Great Britain: A national cohort study. Lancet Oncol. 2008. 9: 239-46
8. Soudry E, Psaltis AJ, Lee KH, Vaezafshar R, Nayak JV, Hwang PH. Complications associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015. 125: 80-5
9. Soyka MB, Serra C, Regli L, Meier E, Holzmann D. Long-term olfactory outcome after nasoseptal flap reconstructions in midline skull base surgery. Am J Rhinol Allergy. 2017. 31: 334-7
10. Wengier A, Ram Z, Warshavsky A, Margalit N, Fliss DM, Abergel A. Endoscopic skull base reconstruction with the nasoseptal flap: Complications and risk factors. Eur Arch Otorhinolaryngol. 2019. 276: 2491-8
11. Zang X, Wang EW, Wei H, Shi J, Snyderman CH, Gardner PA. Anatomy of the posterior septal artery with surgical implications on the vascularized pedicled nasoseptal flap. Head Neck. 2015. 37: 1470-6