- Division of Neurosurgery, Department of Neurosciences, Reproductive and Odontostomatological Sciences, “Federico II” University, Naples, Italy.
Correspondence Address:
Giuseppe Corazzelli, Division of Neurosurgery, Department of Neurosciences, Reproductive and Odontostomatological Sciences, “Federico II” University, Naples, Italy.
DOI:10.25259/SNI_114_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Giuseppe Mariniello1, Sergio Corvino1, Giuseppe Corazzelli1, Francesco Maiuri1. Cervical epidural abscess complicated by a pharyngoesophageal perforation after anterior cervical spine surgery for subaxial spondylodiscitis. 24-Mar-2023;14:102
How to cite this URL: Giuseppe Mariniello1, Sergio Corvino1, Giuseppe Corazzelli1, Francesco Maiuri1. Cervical epidural abscess complicated by a pharyngoesophageal perforation after anterior cervical spine surgery for subaxial spondylodiscitis. 24-Mar-2023;14:102. Available from: https://surgicalneurologyint.com/surgicalint-articles/12211/
Abstract
Background: The anterior approach to the cervical spine is safe and effective, but not without risks. The pharyngoesophageal perforation (PEP) is a rare but potentially life-threatening complication of this surgical route. A prompt diagnosis and adequate treatment are crucial for the prognosis; nevertheless, there is no unique consent about the best management.
Case Description: A 47-year-old woman was referred to our neurosurgical unit for clinical and neuroradiological signs of multilevel cervical spine spondylodiscitis, which was conservatively treated with long-term antibiotic therapy and cervical immobilization after computed tomography-guided biopsy. Nine months later, when the infection was resolved, the patient underwent C3–C6 spinal fusion with anterior plate and screws through anterior approach to the cervical spine for degenerative vertebral changes causing severe myelopathy, and C5– C6 retrolisthesis with instability. Five days after surgical procedure, the patient developed a pharyngoesophageal-cutaneous fistula, detected through wound drainage, and confirmed by swallowing contrast study, without systemic signs of infection. The PEP was conservatively treated, with antibiotic therapy and parenteral nutrition, and it was monitored through seriate swallowing contrast and magnetic resonance studies up to the complete resolution.
Conclusion: The PEP is a potentially fatal complication of the anterior cervical spine surgery. We suggest an accurate intraoperative control of the pharyngoesophageal’s tract integrity at the end of the surgical procedure and a longtime follow-up, because the risk of occurrence is up to several years after surgery.
Keywords: Anterior cervical spine surgery, Esophageal fistula, Pharyngoesophageal perforation, Cervical spondylodiscitis
INTRODUCTION
The anterior cervical spine approach is the most common performed surgical procedure to the anterior cervical spine, mainly for median and neuroforaminal diseases. Although routinely performed, safe and effective, as each other surgical technique, it has its pro and cons, and it is not without risks. Complications related to this surgical route in decreasing order of incidence include dysphagia, postoperative hematoma, worsening of previous myelopathy, symptomatic vocal cord palsy, cerebrospinal fluid fistula, wound infection, worsening of previous radiculopathy, Horner’s syndrome, respiratory failure, pharyngoesophageal perforation (PEP), and instrumentation failure[
The PEP is a rare (incidence ranges from 0.02% to 1.62%)[
It may occur during the surgery or in the postoperative period, up to several years after the surgical procedure.
Although its mortality rate is low (range from 12% to 20%),[
Due to the few reported cases in the literature, there are no defined guidelines about the best management of this complication, and strategies range from conservative treatment to the surgery.[
Here, we report a case of cervical epidural abscess complicated by a PEP occurred after cervical anterior stabilization without discectomy through anterior microsurgical approach to the cervical spine, for severe myelopathy due to multilevel degenerative changes after spondylodiscitis and retrolisthesis with instability.
CASE REPORT
A 47-year-old female complained a month-history of fever, intense neck pain, and progressive motor weakness at upper and lower limbs, with gait disturbance, not responsive to pharmacological conservative treatment. A computed tomography (CT) of the cervical spine showed a median and left paramedian discal herniation at C4–C5 level. A cervical spine magnetic resonance (MRI) showed a severe spondylodiscitis at C4, C5, and C6 levels with mass effect on the spinal cord, where signs of myelopathy were evident, and on the nerve roots.
The neurological examination at admission revealed severe spastic tetraparesis with preservation of proximal shoulder and hip movements, loss of movements of the distal segments of both arms and legs, and impossibility of standing and walking.
Blood cultures, Wright test, and tuber test were negative, while the Widal test was positive for antigen H of Salmonella paratyphi, expression of previous infection. Intravenous corticosteroid therapy was administered.
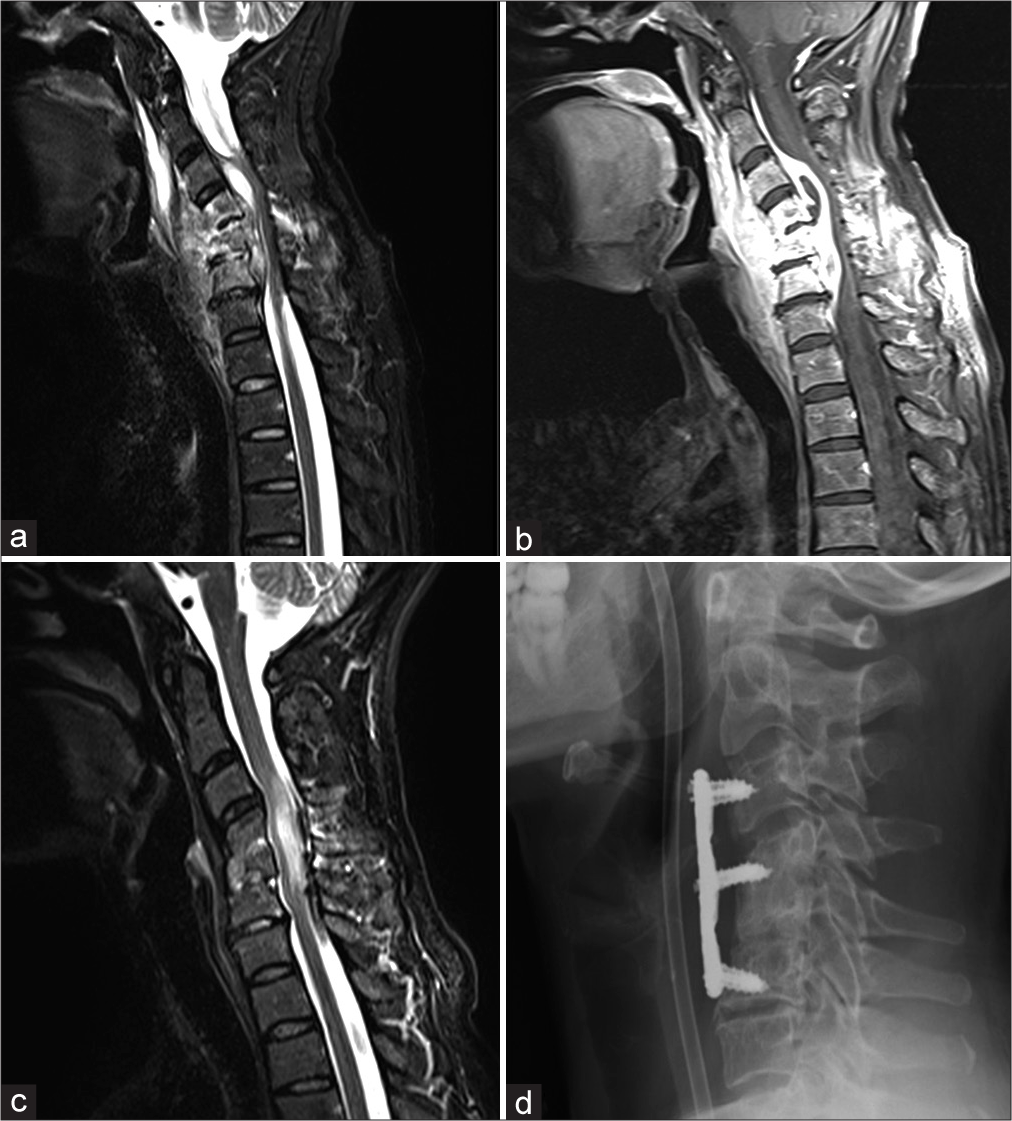
A cervical spine contrast-enhanced MRI contrast showed a marked abnormality at the C4–C5–C6 levels, with fragmentation and destruction of the endplates resulting in focal kyphosis. Dense material on the epidural space, mainly on the ventral side, suggestive of epidural abscess caused severe mass effect on the spinal cord, which appeared markedly compress and posteriorly displaced. Intense and diffuse contrast enhancement of the paravertebral soft tissues from C2 to T1 was also found [
Figure 1:
Cervical spine magnetic resonance imaging (MRI), sagittal sequences, contrast-enhanced T1-weighted (a), and T2-weighted (b) marked abnormality of the C4–C5–C6 vertebral bodies, with fragmentation and destruction of the endplates resulting in focal kyphosis. Dense material on the epidural space, mainly on the ventral side, suggestive of epidural abscess causing mass effect on the pharyngoesophageal tract posterior wall was detected. The infection also involved the surrounding soft paravertebral tissues. Severe mass effect on the spinal cord, which appears markedly compress and posteriorly displaced, was also evident. Postcontrast cervical spine MRI, T1-weighted sequence (c) 8 months after discharge and after prolonged antibiotic therapy. The inflammatory process was resolved resulting in multilevel degenerative changes. Severe myelopathy signal spanning from C3 to C6 level persisted. Cervical radiogram, sagittal projection (d). The correct position of the stabilization system composed by a titanium plate and six screws was evident.
In the following months, the neurological conditions moderately improved, laboratories and neuroradiological examinations showed the progressive resolution of the infectious disease but resulting in multilevel degenerative changes with severe myelopathy [
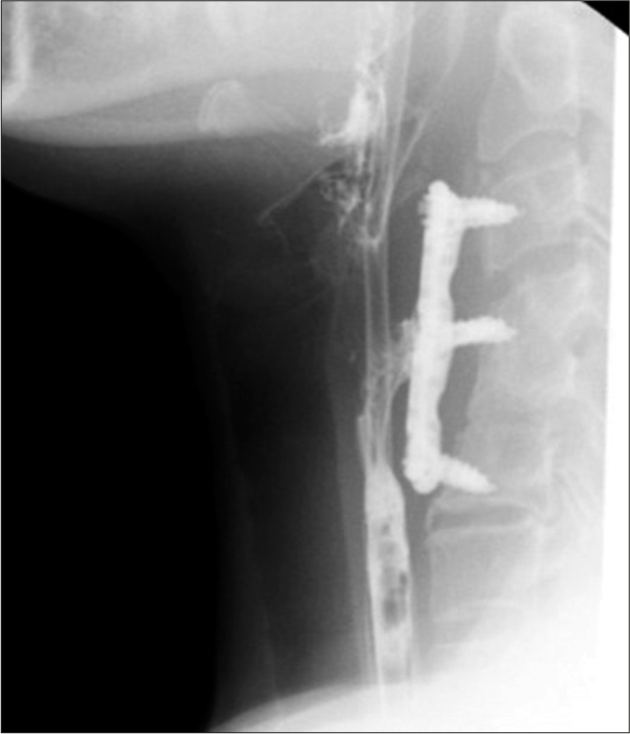
Nine months after the discharge, once the spinal inflammation was resolved, the patient was readmitted to our department, and through anterior approach to the cervical spine, after initial debridement of the subcutaneous tissues, with the pharyngoesophageal complex which appeared tenaciously adherent to the prevertebral fascia and bone and which required careful microsurgical dissection, she underwent C3– C6 anterior cervical stabilization with titanium low profile plate and screws. A postoperative radiogram confirmed the correct position of the implant [
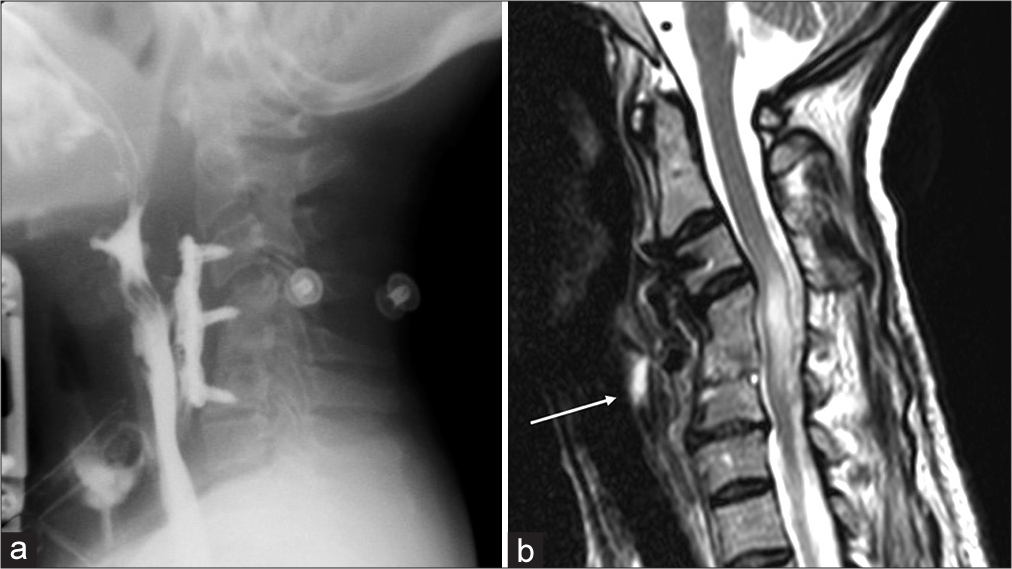
Figure 2:
Barium-swallow study. (a) It was possible to appreciate the pharyngoesophageal perforation through a contrast medium leakage at the pharyngoesophageal junction. Magnetic resonance of the cervical spine, T2-weighted sequence. (b) It is possible to appreciate the persistence of the myelopathy signal and the pharyngoesophageal-cutaneous fistula (white arrow).
The patient was discharged on POD13 with absence of leak on clinical and radiological evaluation, and with nutrition per os.
DISCUSSION
The present case is very interesting for several aspects, including the initial choice of a conservative management, despite the large epidural abscess with severe neurological deterioration, secondly, the occurrence of a pharyngoesophageal-cutaneous fistula and its conservative management, and finally the complete remission of both neurological and esophageal symptoms and signs.
Pyogenic spinal infection more often affects the thoracic and lumbar spine, less frequently the cervical region; nevertheless, the involvement of the cervical region is associated to higher mortality rate (21% vs. 3.6%).[
The spinal cord is very sensitive and small compressions or mass effect from the epidural empyema can lead to severe myelopathy.
Infections of the cervical spine are rare and treatment options are various;[
Considering the timing of treatment, some authors recommend conservative therapy for spinal infections up to 6 weeks[
In regard of the modality of surgical treatment, several authors suggest the use of titanium mesh cages with or without posterior instrumentations;[
Conservative treatment, comprising long-term antibiotics combined with bed rest and/or an orthosis, seems to be the first line of treatment for pyogenic spondylodiscitis; surgery is indicated in cases of compression of neurological structures, pain worsening, persisting infection on imaging, failure of conservative treatment, spinal deformity, or neurological deteriorating.
In our case, we opted for an initial conservative treatment, consisting in targeted systemic antibiotic therapy for 3 months and cervical spine immobilization. During this period, the patient was monitored through laboratory and radiological examinations, and ambulatory controls. The neurological clinical conditions progressively improved in relationship with the resolution of the spinal infection and the related mass effect from epidural abscess.
The ratio of our strategy to delay the surgery was based on avoiding the risk of microbial colonization of the stabilization system and the consequent persistent infection.
The PEP is a rare (incidence ranges from 0.02% to 1.62%)[
Only 159 cases of PEP following anterior cervical spine surgery have been reported in a recent literature review.[
The cervical spinal segments between C3 and C6 levels, for their anatomical relationship with the cricoid, are the most susceptible to the PEP: the hardness of the cricoid cartilage in fact, exercises some pressure’s degree on the posterior wall of the pharyngoesophageal tract during each swallowing act. This predisposing anatomical factor, associated to the incorrect placement of the fixation’s system or hardware displacement, may favor the PEP due to decubitus.[
The PEPs may be classified as early and delayed, if the interval between the surgical procedure and the diagnosis of this complication is under or over 30 days, respectively.[
Our patient underwent contrast swallow to detect the site, size, and morphology of PEP and X-ray and MRI to evaluate the spine fusion and stability, and the placement of the implant; the endoscopic exploration was not necessary, because already from the radiological examinations, we could appreciate no penetration of the implant into the esophagus.
Due to the paucity of literature on PEPs, defined guidelines of management are not present; reported options include conservative and surgical treatments. Among the 159 reported cases in literature,[
Surgical treatment consists of an initial step, in which wound the debridement and drainage, if infection is present, are made, followed by PEP repair through suture of the defect and reinforcement with muscle flap. If the fusion system is infected or migrate, or plate/screw decubitus is present, the removal of the fixation device is recommended.[
Our patient was treated conservatively due to minimal leak (<1 cm) and absence of local and systemic infection signs. We consider that the best management mainly depends on the entity of the perforation and the local condition. Large fistulas with significant inflammatory collection should be operated on. On the other hand, for small perforations, we suggest a conservative management.
CONCLUSION
The PEP is a rare but life-threatening complication related to the anterior approach to the cervical spine. A tempestive diagnosis and appropriate treatment are mandatories. Defined guidelines of management are not present; the treatment should be tailored according to the size, site, and the morphology of the defect. We suggest an accurate intraoperative control of the pharyngoesophageal’s tract integrity at the end of surgical procedure and a longtime follow-up due to the risk of occurrence up to several years after surgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK. 2015 infectious diseases society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015. 61: e26-46
2. Burkhardt BW, Müller SJ, Wagner AC, Oertel JM. Anterior cervical spine surgery for the treatment of subaxial cervical spondylodiscitis: A report of 30 consecutive patients. Neurosurg Focus. 2019. 46: E6
3. Curry WT, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: Clinical presentation, management, and outcome. Surg Neurol. 2005. 63: 364-71 discussion 371
4. Dakwar E, Uribe JS, Padhya TA, Vale FL. Management of delayed esophageal perforations after anterior cervical spinal surgery. J Neurosurg Spine. 2009. 11: 320-5
5. Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006. 355: 2012-20
6. Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004. 26: 285-91
7. Epstein NE. A review of complication rates for anterior cervical diskectomy and fusion (ACDF). Surg Neurol Int. 2019. 10: 100
8. Ghirelli M, Molinari G, Rosini M, De Iure F, Gasbarrini A, Mattioli F. Pharyngo-esophageal perforations after anterior cervical spine surgery: Management and outcomes. World Neurosurg. 2020. 139: e463-73
9. Ghobrial GM, Franco D, Theofanis T, Margiotta PJ, Andrews E, Wilson JR. Cervical spondylodiscitis: Presentation, timing, and surgical management in 59 patients. World Neurosurg. 2017. 103: 664-70
10. Halani SH, Baum GR, Riley JP, Pradilla G, Refai D, Rodts GE. Esophageal perforation after anterior cervical spine surgery: A systematic review of the literature. J Neurosurg Spine. 2016. 25: 285-91
11. Heyde CE, Boehm H, El Saghir H, Tschöke SK, Kayser R. Surgical treatment of spondylodiscitis in the cervical spine: A minimum 2-year follow-up. Eur Spine J. 2006. 15: 1380-7
12. Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: A ten-year perspective. Neurosurgery. 1990. 27: 177-84
13. Karadimas EJ, Bunger C, Lindblad BE, Hansen ES, Høy K, Helmig P. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop. 2008. 79: 650-9
14. Karikari IO, Powers CJ, Reynolds RM, Mehta AI, Isaacs RE. Management of a spontaneous spinal epidural abscess: A single-center 10-year experience. Neurosurgery. 2009. 65: 919-23 discussion 923-14
15. Korovessis P, Repantis T, Iliopoulos P, Hadjipavlou A. Beneficial influence of titanium mesh cage on infection healing and spinal reconstruction in hematogenous septic spondylitis: A retrospective analysis of surgical outcome of twenty-five consecutive cases and review of literature. Spine (Phila Pa 1976). 2008. 33: E759-67
16. Linhardt O, Matussek J, Refior HJ, Krödel A. Long-term results of ventro-dorsal versus ventral instrumentation fusion in the treatment of spondylitis. Int Orthop. 2007. 31: 113-9
17. Moletta L, Pierobon ES, Salvador R, Volpin F, Finocchiaro FM, Capovilla G. Pharyngo-esophageal perforation following anterior cervical spine surgery: A single center experience and a systematic review of the literature. Global Spine J. 2021. 12: 719-31
18. Mondorf Y, Gaab MR, Oertel JM. PEEK cage cervical ventral fusion in spondylodiscitis. Acta Neurochir (Wien). 2009. 151: 1537-41
19. Nanda A, Sharma M, Sonig A, Ambekar S, Bollam P. Surgical complications of anterior cervical diskectomy and fusion for cervical degenerative disk disease: A single surgeon’s experience of 1,576 patients. World Neurosurg. 2014. 82: 1380-7
20. Pereira CE, Lynch JC. Spinal epidural abscess: An analysis of 24 cases. Surg Neurol. 2005. 63: S26-29
21. Perrone O, Tassi V, Mattioli B, Daddi N, Uneddu M, Borghesi I. Pharyngo-oesophageal perforation following anterior cervical discectomy and fusion: Management and results. Eur J Cardiothorac Surg. 2017. 51: 160-8
22. Pojskić M, Carl B, Schmöckel V, Völlger B, Nimsky C, Saβ B. Neurosurgical management and outcome parameters in 237 patients with spondylodiscitis. Brain Sci. 2021. 11: 1019
23. Pola E, Taccari F, Autore G, Giovannenze F, Pambianco V, Cauda R. Multidisciplinary management of pyogenic spondylodiscitis: Epidemiological and clinical features, prognostic factors and long-term outcomes in 207 patients. Eur Spine J. 2018. 27: 229-36
24. Ruf M, Stoltze D, Merk HR, Ames M, Harms J. Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine (Phila Pa 1976). 2007. 32: E275-80
25. Rutges JP, Kempen DH, van Dijk M, Oner FC. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: A systematic literature review. Eur Spine J. 2016. 25: 983-99
26. Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: A potentially dramatic disease. J Spinal Disord Tech. 2002. 15: 110-7
27. Shad A, Shariff S, Fairbank J, Byren I, Teddy PJ, Cadoux-Hudson TA. Internal fixation for osteomyelitis of cervical spine: The issue of persistence of culture positive infection around the implants. Acta Neurochir (Wien). 2003. 145: 957-60 discussion 960
28. Shousha M, Boehm H. Surgical treatment of cervical spondylodiscitis: A review of 30 consecutive patients. Spine (Phila Pa 1976). 2012. 37: E30-6
29. Shousha M, Heyde C, Boehm H. Cervical spondylodiscitis: Change in clinical picture and operative management during the last two decades. A series of 50 patients and review of literature. Eur Spine J. 2015. 24: 571-6
30. Sørensen P. Spinal epidural abscesses: Conservative treatment for selected subgroups of patients. Br J Neurosurg. 2003. 17: 513-8
31. Tasiou A, Giannis T, Brotis AG, Siasios I, Georgiadis I, Gatos H. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg. 2017. 3: 444-59
32. Taylor DG, Buchholz AL, Sure DR, Buell TJ, Nguyen JH, Chen CJ. Presentation and outcomes after medical and surgical treatment versus medical treatment alone of spontaneous infectious spondylodiscitis: A systematic literature review and meta-analysis. Global Spine J. 2018. 8: 49S-58S
33. Tew JM, Mayfield FH. Complications of surgery of the anterior cervical spine. Clin Neurosurg. 1976. 23: 424-34
34. Urrutia J, Zamora T, Campos M. Cervical pyogenic spinal infections: Are they more severe diseases than infections in other vertebral locations?. Eur Spine J. 2013. 22: 2815-20
35. Valancius K, Hansen ES, Høy K, Helmig P, Niedermann B, Bünger C. Failure modes in conservative and surgical management of infectious spondylodiscitis. Eur Spine J. 2013. 22: 1837-44
36. Yee TJ, Swong K, Park P. Complications of anterior cervical spine surgery: A systematic review of the literature. J Spine Surg. 2020. 6: 302-22