- Department of Neurosurgery, Hue University Hospital, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam
- Department of Histology, Embryology, Pathology and Forensic Medicine, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam
Correspondence Address:
Van Tri Truong, Department of Neurosurgery, Hue University Hospital, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam.
DOI:10.25259/SNI_615_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bao Quoc Nguyen1, Duc Duy Tri Tran1, Thuan Cong Dang2, Thi Dang Mai1, Hai Duong Pham1, Van Tri Truong1. Cervical intra-extradural meningioma with en-plaque, dumbbell-shaped, and an unusual calcified pattern in a young patient. 06-Sep-2021;12:454
How to cite this URL: Bao Quoc Nguyen1, Duc Duy Tri Tran1, Thuan Cong Dang2, Thi Dang Mai1, Hai Duong Pham1, Van Tri Truong1. Cervical intra-extradural meningioma with en-plaque, dumbbell-shaped, and an unusual calcified pattern in a young patient. 06-Sep-2021;12:454. Available from: https://surgicalneurologyint.com/surgicalint-articles/11087/
Abstract
Background: Most spinal meningiomas primarily grow in the intradural extramedullary location. Epidural meningiomas are uncommon; if detected, they usually coexist with intradural lesions. They inhere more aggressive and invasive characteristics compared with their counterparts inside the dura.
Case Description: We report a 22-year-old female who was admitted to the hospital with weakness and numbness in both lower limbs. Her cervical magnetic resonance imaging revealed an en-plaque and dumbbell-shaped lesion located from C5 to C8. After gadolinium injection, the whole mass was enhanced and unveiled two portions: intradural and extradural. The bone window of the computed tomography scan revealed calcification inside the lesion. The patient underwent tumor removal surgery. The pathology findings showed a psammomatous meningioma. After 6 months of surgery, the patient has been able to walk with walkers.
Conclusion: We should consider spinal meningioma as a differential diagnosis when encountering an extradural lesion in the cervical region. The optimal surgical treatment for young patient with epidural meningiomas is radical surgery with dura attachment removal.
Keywords: Calcification, Cervical spine, Dumbbell shaped, En plaque, Epidural meningioma
INTRODUCTION
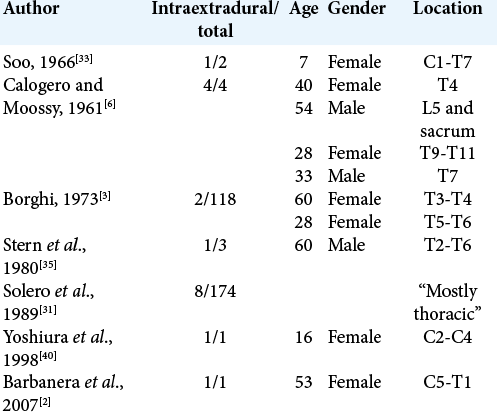
Meningiomas are common lesions in the spine, covering about 25% of all spinal cord tumors.[
Spinal meningiomas originate from arachnoid cap cells lying within the arachnoid membrane.[
In general, epidural meningiomas have been categorized into three different growth patterns: en plaque, dumbbell shaped, and fusiform.[
CASE REPORT
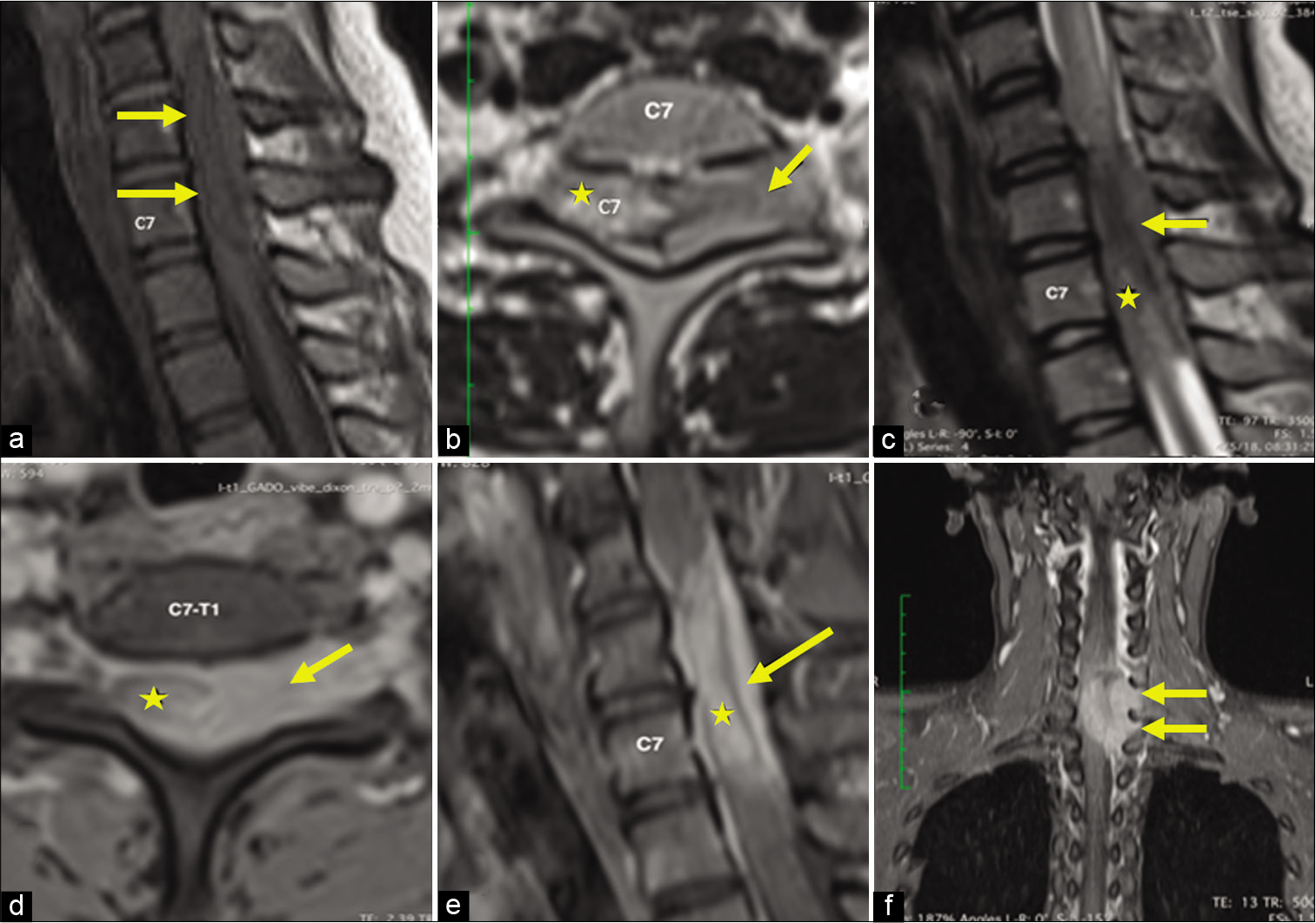
A 22-year-old female was admitted to the hospital with weakness and numbness in both lower limbs. On neurological evaluation, sensory examination showed reduced pain and temperature sensations below dermatome T1 and loss of vibration sensation and proprioception in all extremities; motor examination showed 2/5 muscle strength in lower extremities and normal upper extremities strength except for a mild weakness at right fingers flexion. She also had global hyperreflexia in all extremities; ankle clonus and Babinski’s sign are positive on both sides. Her cervical magnetic resonance imaging (MRI) revealed a lesion located from C5 to C8 hypointense compared to the spinal cord on the T1-weighted (T1W) image and slightly hyperintense on the T2-weighted (T2W) image. After gadolinium injection, the whole mass was enhanced and unveiled two portions: intradural and extradural [
Figure 1:
Magnetic resonance imaging of the cervical spine without gadolinium reveals a lesion which is hypointense (arrows) on the T1W sagittal (a) image. The lesion appears lightly hyperintense on the T2W axial (b) and sagittal (c) image with an intradural (star) and extradural (arrow) portion. Both components are enhanced after gadolinium injection on the T1W axial (d) and sagittal (e) image, which expands through the foramen on the T1W coronal image (f) with a dumbbell-shaped pattern.
DISCUSSION
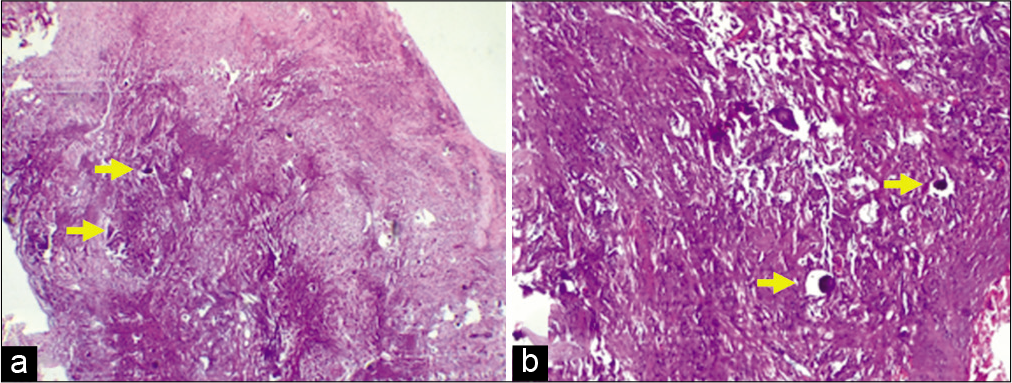
The histologic image that makes meningiomas standout among central nervous system tumors is psammoma bodies.[
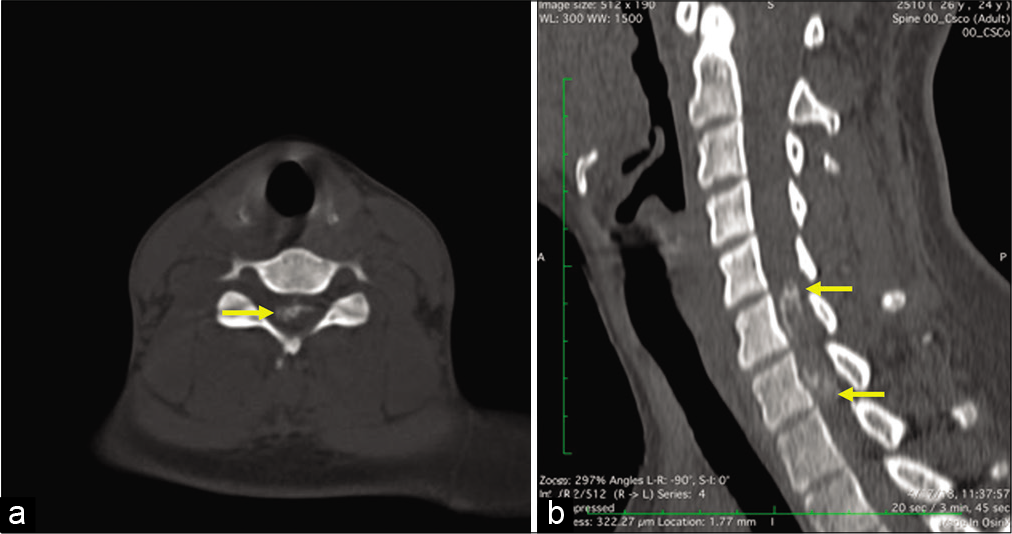
On MRI, spinal meningiomas normally show isointense to the spinal cord on T1W and T2W, and hypointense on T2W if calcified. T1W with contrast illustrates a prominent and homogeneous enhancement. Dural tails could usually be seen in this sequence. On CT scan, we could observe calcification which may assist a diagnosis of meningioma. However, it is only found in 1.0–4.6% of all spinal meningiomas.[
Based on the location and the features of the lesion, we were concerned about either an intraextradural lesion, an extended intramedullary tumor, or metastasis. Our differential diagnoses include metastasis, ependymoma, glioblastoma multiforme, meningioma, and nerve sheath tumors (schwannoma/neurofibroma).
In spite of a negative metastatic work-up, physicians should always raise concerns about a cancerous potential when confronting an extradural lesion regardless of age. Metastases can spread throughout the vertebrae, epidural space, or spinal cord.[
The intradural portion has raised the concern about an extension of an intramedullary tumor. Spinal ependymomas arise from ependymal cells lining the central canal; therefore, they are strictly intramedullary in location. Due to their specific origin, they usually widen the spinal cord on the imaging. MRI reveals isointense to hypointense that would be seen on T1W and hyperintense on T2W. T1W with contrast shows a strong enhancement and is somewhat inhomogeneous. They classically have the “cap sign” (a hypointense hemosiderin rim on T2-weighted images), indicating a hemorrhage in 20–33% of cases. In contrast to intracranial ependymomas, calcification is uncommon in this entity.[
Despite the fact that calcified spinal glioblastoma multiforme is extremely rare,[
Nerve sheath tumors are characterized by isointense or hypointense on T1W and may be heterogeneously hyperintense on T2W. The heterogeneous enhanced dumbbell-shaped expansion on the T1W with contrast has posed the likelihood of a nerve sheath tumor. However, calcified schwannomas/neurofibromas are rare,[
Microscopic calcification is a common finding, but gross appearance is only seen in 1–5% of all spinal meningiomas.[
Most spinal meningiomas are benign tumors; however, when they occur at younger age, the lesions are somewhat more aggressive.[
In recent days, the word “dumbbell shaped” does not necessarily solely illustrate an hourglass figure, it could, however, broadly refer to a tumor which has two distinct parts connecting to each other from two different locations (i.e. intradural and epidural) but outside the paravertebral space.[
Management and prognosis
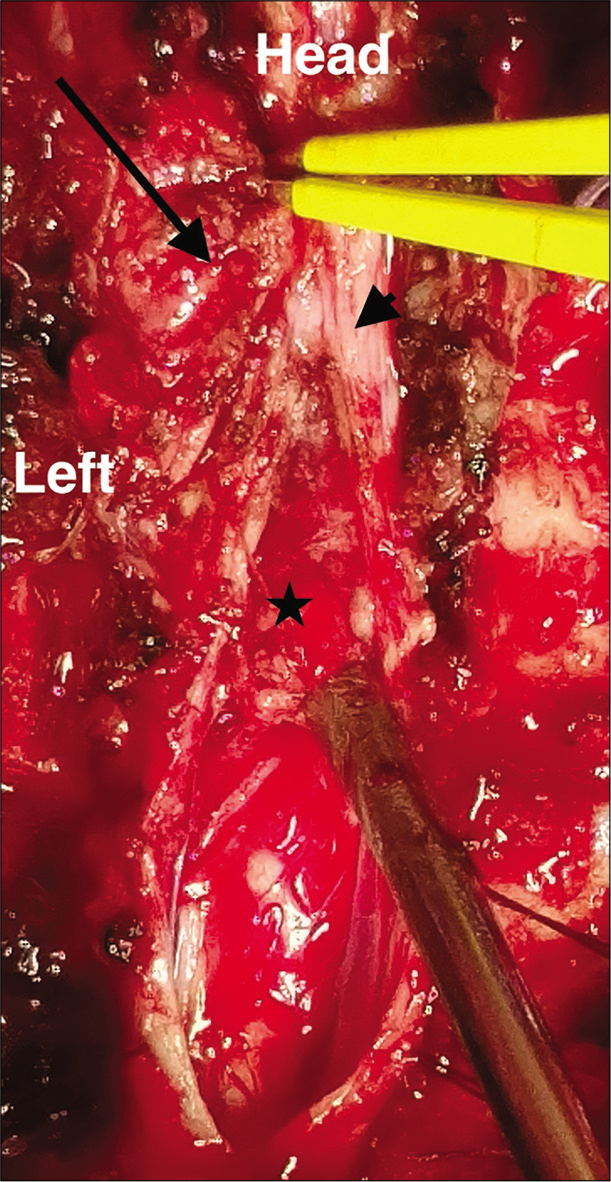
From a surgical viewpoint it is very important to identify exactly the location and characteristics of meningiomas before surgery. As most meningiomas are benign, we can expect a good surgical outcome. However, en-plaque and calcified epidural meningiomas often severely adhere to adjacent tissues, making them extremely hard to be completely removed.[
The arachnoid, in fact, protects the cord from an expansion of meningiomas before the first surgery; from there on, it will inevitably be impaired because of scarring effects.[
In our case, we have extracted the mass in toto followed by dura closure. Among elderly patients, the recurrence rate is generally not being affected by the management of the dural attachment (i.e., resection versus cauterization); this may be due to the latency effect.[
CONCLUSION
Epidural meningioma is uncommon and usually coexists with an intradural lesion; therefore, a careful preoperative imaging evaluation has a critical role in establishing diagnosis and surgical strategy. Due to the longer life expectancy, the optimal treatment for young patients should be radical surgery with dura attachment removal.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
All authors have contributed equally. We thank the patient for allowing us to share her details.
References
1. Alafaci C, Grasso G, Granata F, Salpietro FM, Tomasello F. Ossified spinal meningiomas: Clinical and surgical features. Clin Neurol Neurosurg. 2016. 142: 93-7
2. Barbanera A, Nina P, Serchi E, Ascanio F. Aggressive recurrence of intra-extradural cervico-thoracic meningothelial meningioma. Acta Neurochir (Wien). 2007. 149: 83-6
3. Borghi G. Extradural spinal meningiomas. Acta Neurochir (Wien). 1973. 29: 195-202
4. Buchfelder M, Nomikos P, Paulus W, Rupprecht H. Spinal-thoracic dumbbell meningioma: A case report. Spine. 2001. 26: 1500-4
5. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018. 14: 2161-77
6. Calogero JA, Moossy J. Extradural spinal meningiomas. Report of four cases. J Neurosurg. 1972. 37: 442-7
7. Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE. Spinal meningiomas in patients younger than 50 years of age: A 21-year experience. J Neurosurg. 2003. 98: 258-63
8. Esses SI, Morley TP. Spinal arachnoiditis. Can J Neurol Sci J Can Sci Neurol. 1983. 10: 2-10
9. Frank BL, Harrop JS, Hanna A, Ratliff J. Cervical extradural meningioma: Case report and literature review. J Spinal Cord Med. 2008. 31: 302-5
10. Freidberg SR. Removal of an ossified ventral thoracic meningioma. Case report. J Neurosurg. 1972. 37: 728-30
11. Horrax G, Poppen JL, Wu WQ, Weadon PR. Meningiomas and neurofibromas of the spinal cord: Certain clinical features and end results. Surg Clin North Am. 1949. 29: 659-65
12. Ibrahim AW, Satti MB, Ibrahim EM. Extraspinal meningioma. Case report. J Neurosurg. 1986. 64: 328-30
13. Isobe K, Shimizu T, Akahane T, Kato H. Imaging of ancient schwannoma. AJR Am J Roentgenol. 2004. 183: 331-6
14. Kang JK, Kim MC, Kim DS, Song JU. Effects of tethering on regional spinal cord blood flow and sensory-evoked potentials in growing cats. Childs Nerv Syst. 1987. 3: 35-9
15. Kim MS, Eun JP, Park JS. A dumbbell-shaped meningioma mimicking a schwannoma in the thoracic spine. J Korean Neurosurg Soc. 2011. 50: 264-7
16. Klekamp J, Batzdorf U, Samii M, Bothe HW. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997. 86: 233-40
17. Klekamp J, Samii M. Surgical results for spinal meningiomas. Surg Neurol. 1999. 52: 552-62
18. Koeller KK, Rosenblum RS, Morrison AL. Neoplasms of the spinal cord and filum terminale: Radiologic-pathologic correlation. Radiographics. 2000. 20: 1721-49
19. Lee JW, Lee IS, Choi KU, Lee YH, Yi JH, Song JW. CT and MRI findings of calcified spinal meningiomas: correlation with pathological findings. Skeletal Radiol. 2010. 39: 345-52
20. Levy WJ, Bay J, Dohn D. Spinal cord meningioma. J Neurosurg. 1982. 57: 804-12
21. Lunardi P, Missori P, Franco C, Delfini R, Fortuna A. Hard-rock spinal meningioma. Case report and review of the literature. J Neurosurg Sci. 1992. 36: 243-6
22. Matsumoto S, Hasuo K, Uchino A, Mizushima A, Furukawa T, Matsuura Y. MRI of intradural-extramedullary spinal neurinomas and meningiomas. Clin Imaging. 1993. 17: 46-52
23. McCormick PC. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996. 38: 67-74
24. Messori A, Rychlicki F, Salvolini U. Spinal epidural en-plaque meningioma with an unusual pattern of calcification in a 14-year-old girl: Case report and review of the literature. Neuroradiology. 2002. 44: 256-60
25. Milz H, Hamer J. Extradural spinal meningiomas. Neurochirurgia. 1983. 26: 126-9
26. Nowosielski M, Galldiks N, Iglseder S, Kickingereder P, von Deimling A, Bendszus M. Diagnostic challenges in meningioma. Neuro Oncol. 2017. 19: 1588-98
27. Rasmussen TB, Kernoilan JW, Adson AW. Pathologic classification, with surgical consideration, of intraspinal tumors. Ann Surg. 1940. 111: 513-30
28. Rogers L. Tumours involving the spinal cord and its nerve roots. Ann R Coll Surg Engl. 1955. 16: 1-29
29. Shah LM, Salzman KL. Imaging of spinal metastatic disease. Int J Surg Oncol. 2011. 2011: 769753
30. Shastin D, Mathew RK, Ismail A, Towns G. Cervical spinal glioblastoma multiforme in the elderly. BMJ Case Rep. 2017. 2017: bcr2016217742
31. Solero CL, Fornari M, Giombini S, Lasio G, Oliveri G, Cimino C. Spinal meningiomas: Review of 174 operated cases. Neurosurgery. 1989. 25: 153-60
32. Solomon DA, Pekmezci M.editors. Pathology of meningiomas. Handbook of Clinical Neurology. Amsterdam, Netherlands: Elsevier; 2020. 169: 87-99
33. Soo LY. Spinal epidural meningioma. South Med J. 1966. 59: 141-4
34. Stechison MT, Tasker RR, Wortzman G. Spinal meningioma en plaque. Report of two cases. J Neurosurg. 1987. 67: 452-5
35. Stern J, Whelan MA, Correll JW. Spinal extradural meningiomas. Surg Neurol. 1980. 14: 155-9
36. Suzuki A, Nakamura H, Konishi S, Yamano Y. Dumbbell-shaped meningioma with cystic degeneration in the thoracic spine: A case report. Spine. 2002. 27: E193-6
37. Tuli J, Drzymalski DM, Lidov H, Tuli S. Extradural En-plaque spinal meningioma with intraneural invasion. World Neurosurg. 2012. 77: 202.e5-13
38. Vloeberghs M, Herregodts P, Stadnik T, Goossens A, D’Haens J. Spinal arachnoiditis mimicking a spinal cord tumor: A case report and review of the literature. Surg Neurol. 1992. 37: 211-5
39. Wu L, Yang T, Deng X, Yang C, Zhao L, Yao N. Spinal extradural en plaque meningiomas: clinical features and long-term outcomes of 12 cases. J Neurosurg Spine. 2014. 21: 892-8
40. Yoshiura T, Shrier DA, Pilcher WH, Rubio A. Cervical spinal meningioma with unusual MR contrast enhancement. AJNR Am J Neuroradiol. 1998. 19: 1040-2
41. Zevgaridis D, Thomé C. Purely epidural spinal meningioma mimicking metastatic tumor: Case report and review of the literature. Spine. 2002. 27: E403-5
42. Zhang LH, Yuan HS. Imaging appearances and pathologic characteristics of spinal epidural meningioma. AJNR Am J Neuroradiol. 2018. 39: 199-204