- Department of Paediatrics, South West Acute Hospital, Enniskillen, County Fermanagh, United Kingdom,
- Department of Radiology, South West Acute Hospital, Enniskillen, County Fermanagh, United Kingdom.
Correspondence Address:
Sarvesh Kutty, Department of Paediatrics, South West Acute Hospital, Enniskillen, County Fermanagh, United Kingdom.
DOI:10.25259/SNI_602_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sarvesh Kutty1, Glen Clarke2, Jayasree Kutty1. Challenges in the pre- and post-natal diagnosis of posterior fossa cysts: A case report and review of historical evolution of descriptive terminologies. 30-Sep-2022;13:449
How to cite this URL: Sarvesh Kutty1, Glen Clarke2, Jayasree Kutty1. Challenges in the pre- and post-natal diagnosis of posterior fossa cysts: A case report and review of historical evolution of descriptive terminologies. 30-Sep-2022;13:449. Available from: https://surgicalneurologyint.com/surgicalint-articles/11901/
Abstract
Background: Radiological diagnoses of posterior fossa cystic abnormalities during antenatal and postnatal periods pose significant challenges as they may have similar early imaging features. Some of the frequently described entities are arachnoid cysts and Dandy-Walker malformations. Blake’s pouch cyst is relatively underdiagnosed. The main aim of the study was to explore these diagnostic challenges in the context of various descriptive terminologies and their prognostic implications.
Methods: We illustrate this through our case, where fetal magnetic resonance imaging (MRI) at 36 weeks gestation showed small right cerebellum without hydrocephalus or hemorrhage. Possible differential diagnoses included Dandy-Walker malformation or posterior fossa malformations, facial hemangiomas, arterial anomalies, cardiac and eye anomalies, sternal clefting, and supraumbilical raphe.
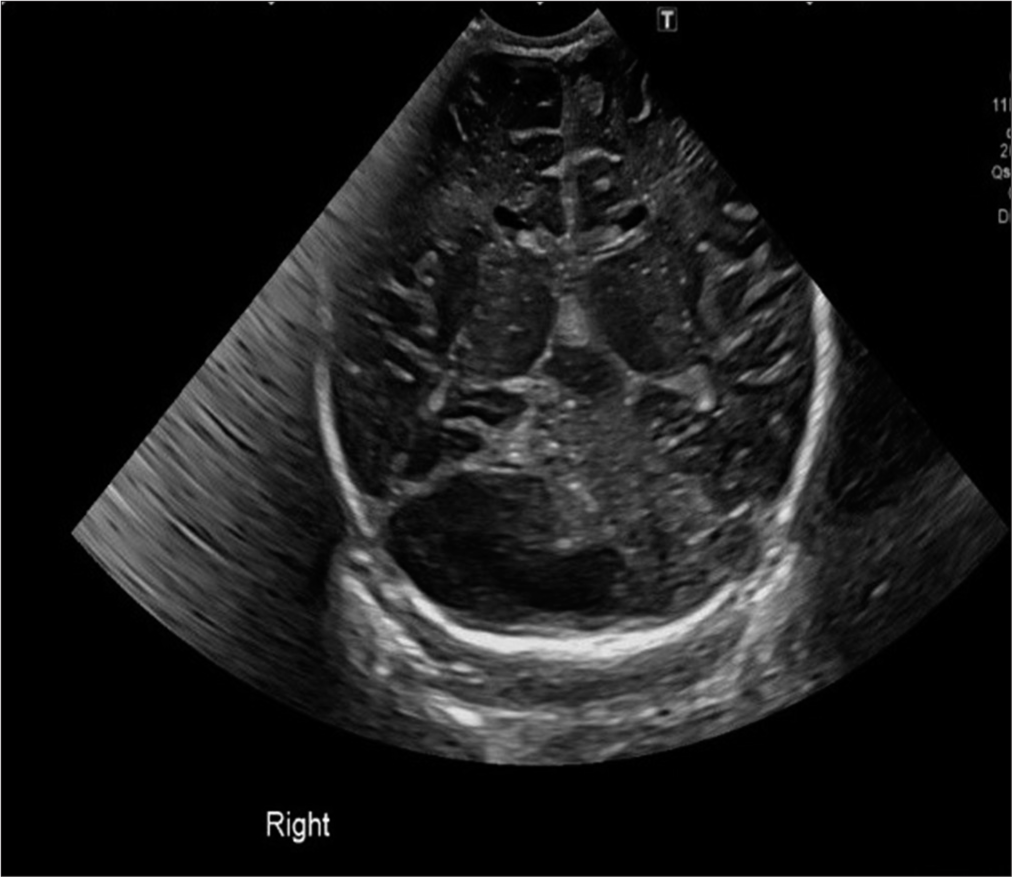
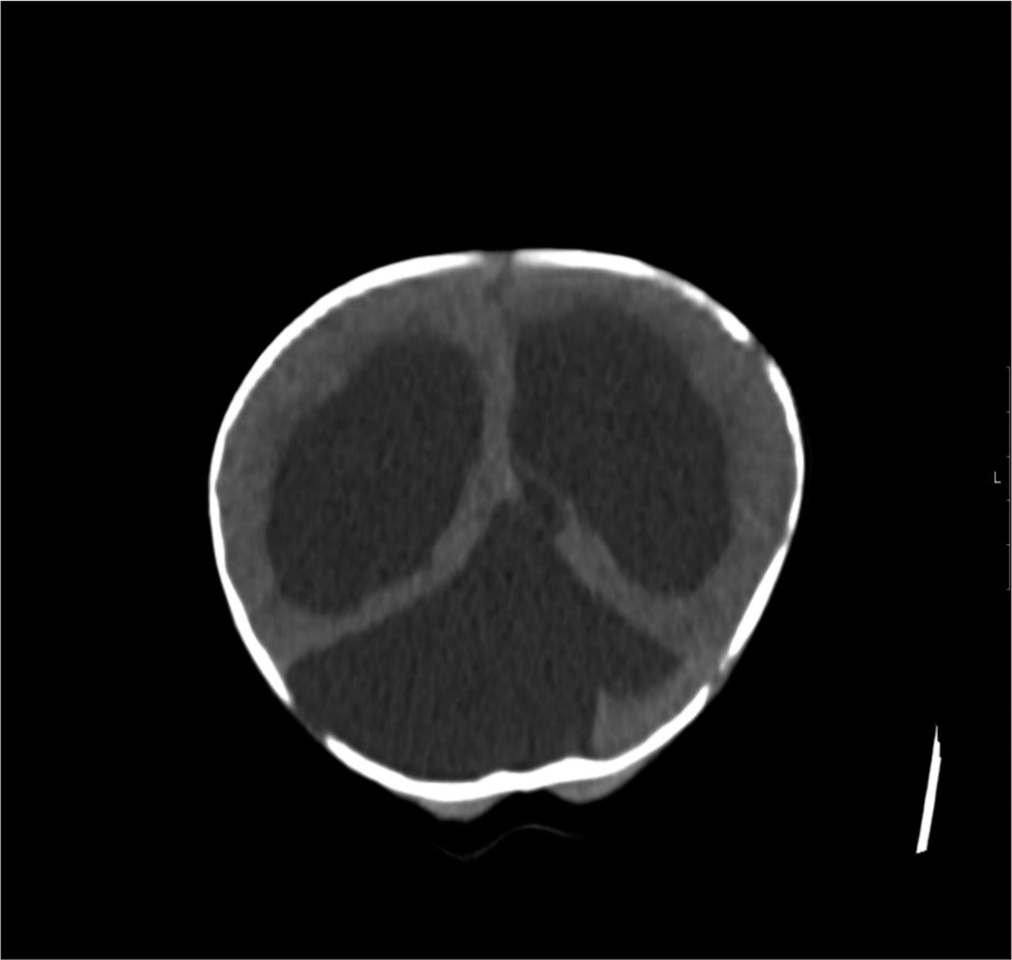
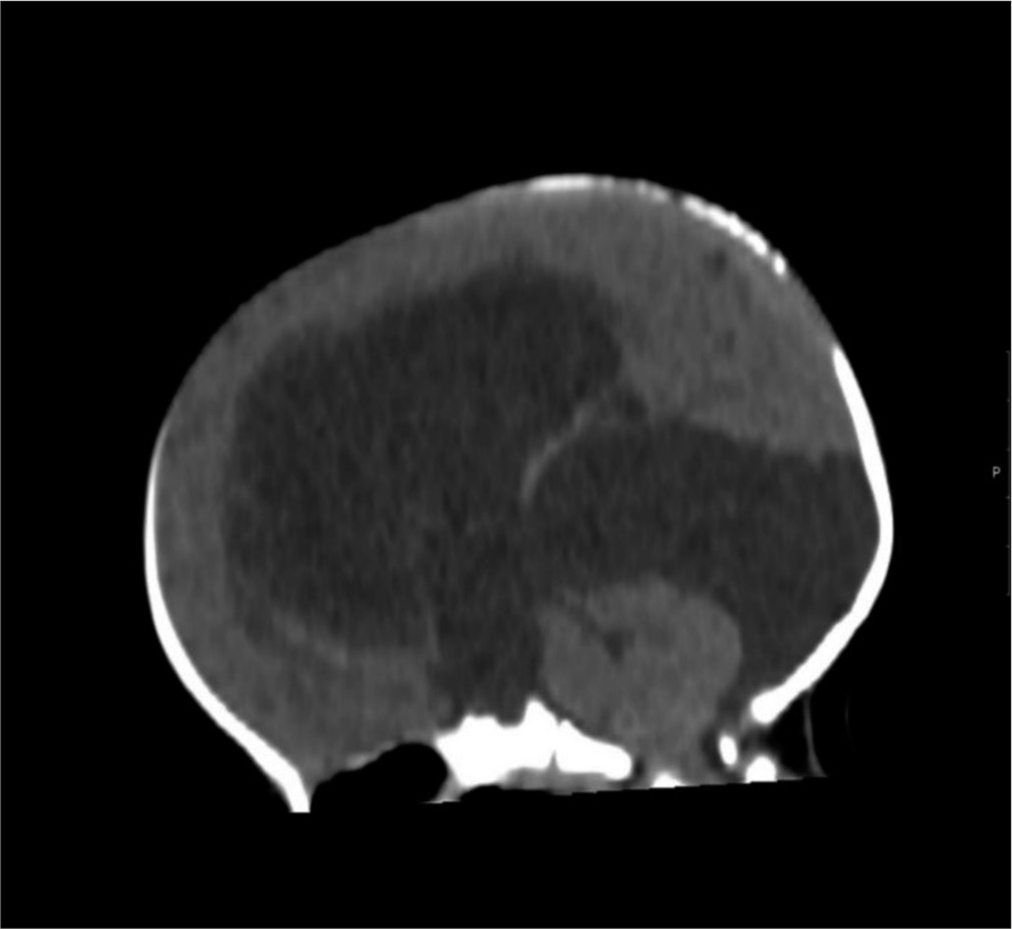
Results: Postnatal sonography noted posterior fossa cyst without hydrocephalus in a normal term infant, who went on to develop symptomatic hydrocephalus by 15 weeks. Computed tomography brain scan confirmed large subtentorial posterior fossa cyst and extensive internal hydrocephalus. Despite emergent ventriculoperitoneal shunt insertion, head circumference continued to rise. MRI scan showed persistent cyst. Subsequently, infant underwent endoscopic fenestration of the cyst with balloon septostomy and now has an age appropriate developmental profile.
Conclusion: There is considerable discordance between antenatal and postnatal neuroimaging findings as highlighted in our case. Diagnostic conundrum here was whether this was an arachnoid or Blake’s pouch cyst. Differentiating between posterior fossa fluid collections is crucial for management, prognosis, and parental counseling. Close postnatal follow-up is essential to avert complications due to acute hydrocephalus.
Keywords: Arachnoid cyst, Blake pouch cyst, Hydrocephalus, Posterior fossa, Retrocerebellar cyst
INTRODUCTION
A range of fetal posterior fossa malformations (PFM) has been reported in one in 5000 live births.[
In recent times, advances in neuroimaging techniques, genetic sequencing, and etiological research have led to precise definition and better classification of congenital abnormalities of posterior fossa.[
Doherty et al.[
CASE DESCRIPTION
A 29-year-old woman had an obstetric anomaly scan at 19+4 weeks of gestation, which noted a low posterior placenta and recalled for a review scan. Following the detection of a posterior fossa anomaly, the patient was referred to fetal medicine specialists for further evaluation with an magnetic resonance imaging (MRI) scan and antenatal counseling. Pregnancy was otherwise unremarkable with no other significant family history.
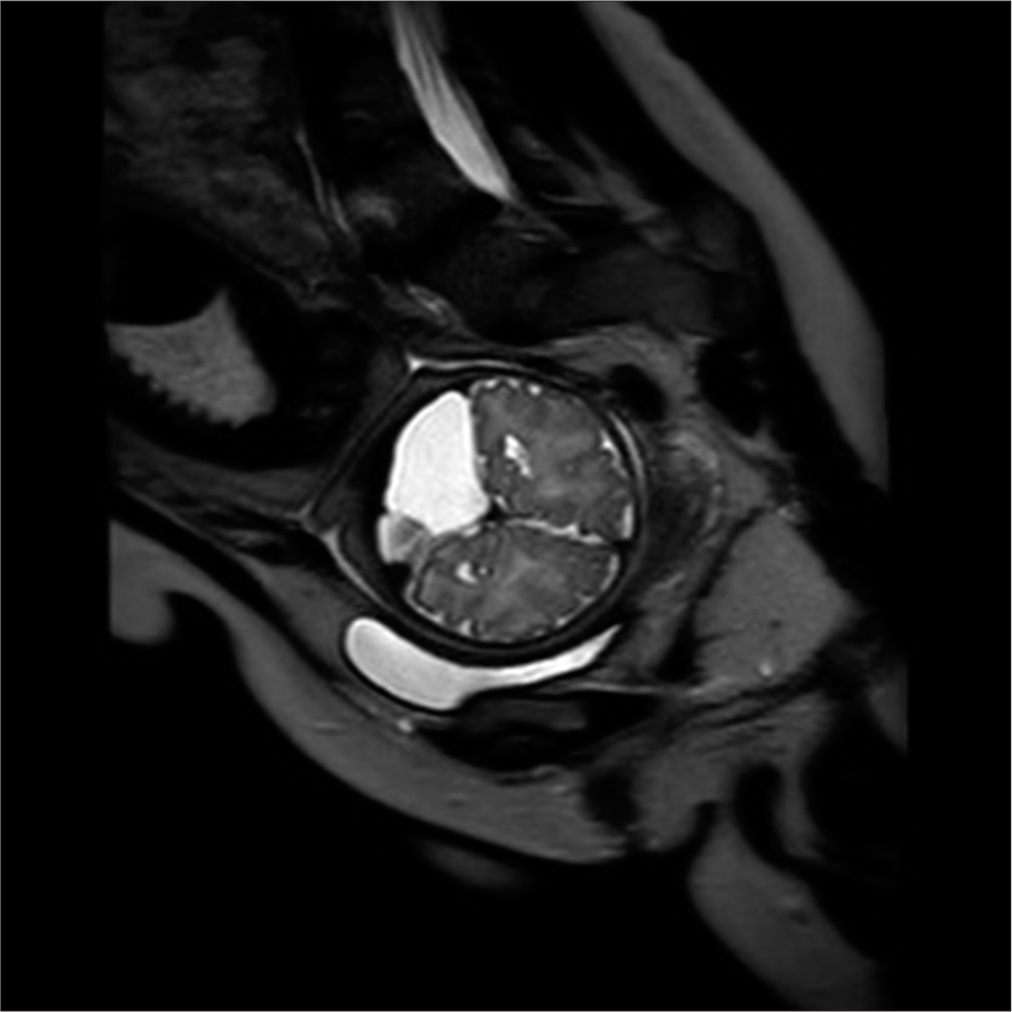
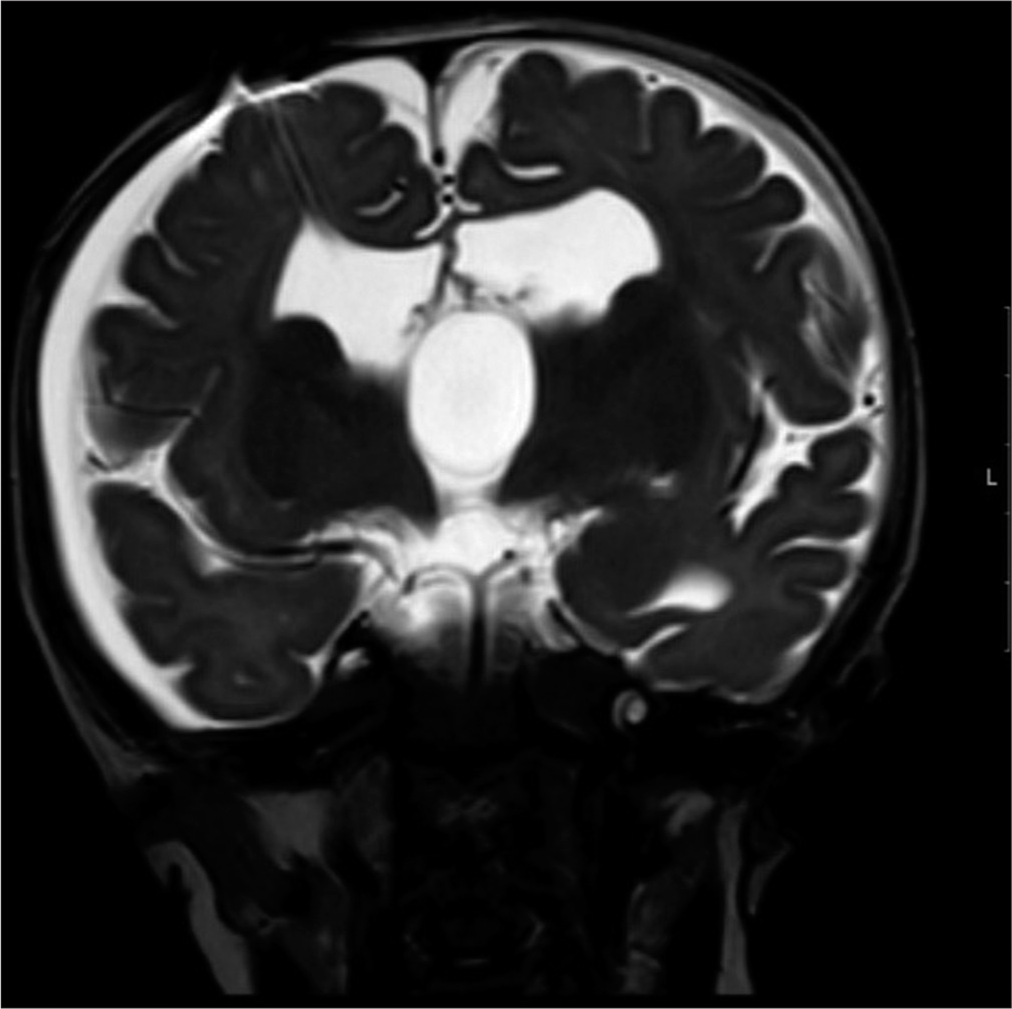
In the fetal MRI scan at 36 weeks gestation, the right cerebellar hemisphere appeared small with prominence of extra axial fluid. Transcerebellar diameter (TCD) was 38 mm (<1st centile). There was suspected mild vermis dysmorphism with a height of 28 mm (>99th centile). The lateral, third, and fourth ventricles were normal in size and shape with normal cavum septum pellucidum. Major intracranial vascular flow voids were grossly unremarkable with appropriate myelination. There was no evidence of acute or old hemorrhage [
Parents were counseled regarding various possible outcomes as the differential diagnoses were unclear based on the available information from the fetal MRI scan. Fetal karyotyping was normal. A female infant was born by normal delivery at 39+2 weeks gestation. Birth weight was 3070 g (9–25th percentile) and occipitofrontal circumference (OFC) was 34 cm (50th percentile). The infant had no dysmorphic features and had a normal neurological examination.
An outpatient follow-up appointment was scheduled in 8 weeks, with health visitor monitoring in the interim. COVID-19 pandemic restrictions during that period impacted follow-up. In retrospect, there were no parental concerns until about 12 weeks of age when they began to notice large head. The infant was then referred to the hospital with increasing head circumference at 15 weeks of age. OFC was 48.8 cm (99.6th percentile) with bulging fontanelle and sun setting appearance of eyes. The infant was otherwise clinically and hemodynamically stable.
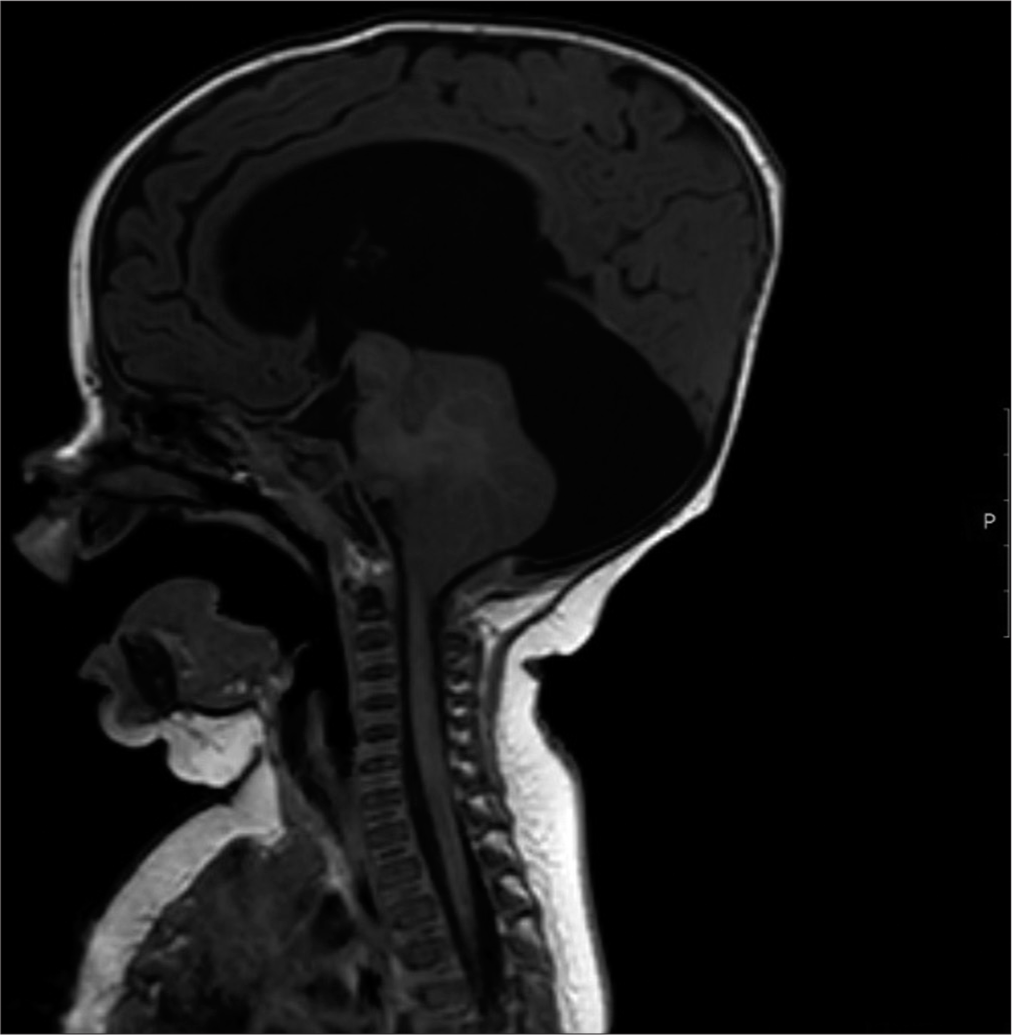
Following transfer to neurosurgical unit, the infant had an emergency right frontal ventriculoperitoneal (VP) shunt insertion and Bactiseal ventricular catheter. The infant did not have an MRI brain scan before this surgery. In hindsight, given the importance of the cyst configuration in relation to the hydrocephalus, this would have been informative. OFC continues to track above the 99.6th percentile. Follow-up MRI brain and cervical cord scan 4 weeks later noted only mild decompression of the supratentorial ventricular system with VP shunt in place [
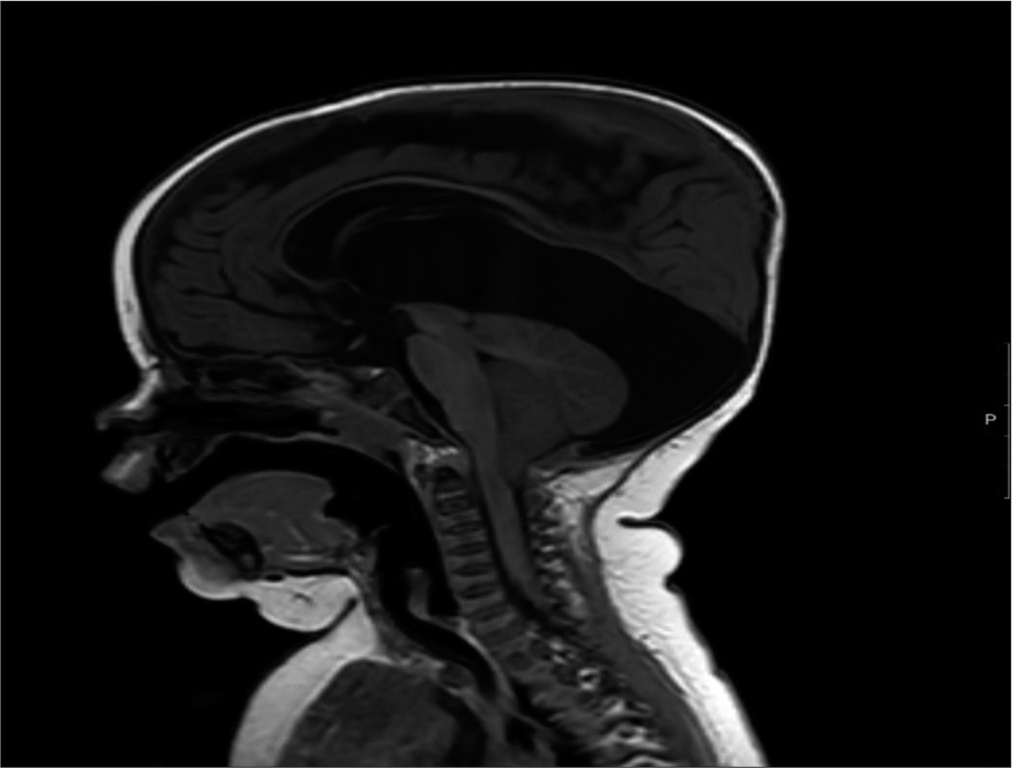
The neurosurgical team carried out an endoscopic fenestration of the cyst, which likely grew and secondarily extended to an area near the pineal region. The previous burr hole was extended laterally after disconnection of the reservoir and ventricular catheter. A 6 mm endoscope was introduced after corticotomy and lateral ventricle was visualized. The large cyst was identified at the foramen of Munroe, which was fenestrated with scissors and balloon septostomy. The ventricular catheter and reservoir were replaced. Postoperatively, there was some resolution of up gaze palsy. Another MRI scan a month later showed the persisting large posterior fossa cyst with new bilateral subdural collections overlying both cerebral hemispheres. This might relate to over shunting of cerebrospinal fluid (CSF) [
The infant’s OFC is tracking above the 99.6th percentile and she has minimal motor skills delay and up gaze palsy. Otherwise, her developmental profile is age appropriate.
DISCUSSION
Our case describes the clinical course of a prenatally detected posterior fossa cyst and its postnatal outcome. Although some sinister pathologies were discussed during counseling, none of these was confirmed later. In this context, we elaborate on the commonly encountered PF cysts and the role of neuroimaging as an invaluable tool in identification as well as prognostication.
Imaging modalities
Ultrasonography is the best initial imaging modality to evaluate fetal posterior fossa with fetal MRI providing excellent additional diagnostic clarity.[
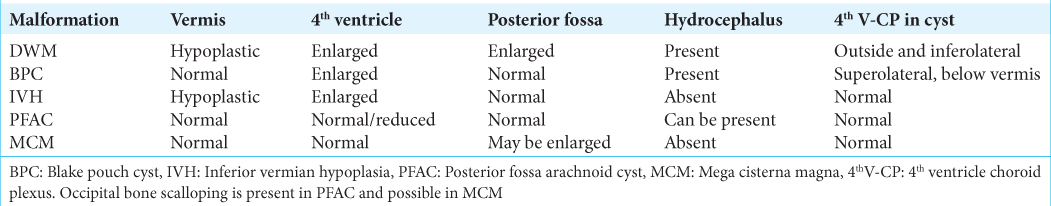
COMMON DIFFERENTIAL DIAGNOSES OF POSTERIOR FOSSA CYSTS
DWM
DWM is the most common PWM with sporadic occurrence and low future recurrence risk of 1–5%.[
MCM
MCM can be an incidental finding where the cisterna magna measures more than 10 mm on midsagittal images. This measurement is arbitrary as some consider it up to 12 mm as normal.[
Arachnoid cyst
When CSF is trapped between duplicated layers of the arachnoid membrane, it forms a fluid-filled cyst called arachnoid cyst. The location can be in the posterior fossa in about 10% of cases in children. They can be retro or lateral cerebellar, above vermis, or anterior to the brain stem.[
Isolated IVH
In isolated IVH, a small vermis partially covers the fourth ventricle. Some authors refer this as Dandy-Walker variant, which is problematic for accurate genetic counseling. In isolation, this has no recurrence risk and has a favorable prognosis in majority of cases. It is worth noting that if this occurs with other genetic conditions such as Joubert syndrome, there is a likelihood of recurrence. Problems with fine motor and receptive language skills are reported with vermian hypoplasia. Prenatal diagnosis is possible after 18–20 weeks of gestation, although ultrasound diagnosis is unreliable. Fetal MRI can delineate partial absence of inferior vermis. Rest of the vermis is normal along with normal cerebellar hemispheres and fourth ventricle. Posterior fossa anatomy and size are otherwise normal.[
BPC
Blake’s pouch is a normal embryonic structure, which arises from the posterior membranous area on the roof of the primitive rhombencephalon. This area enlarges around 4–5th week of gestation and directly communicates with the fourth ventricle. Later, the Blake’s pouch fenestrates to form the foramina of Luschka and Magendie, which is vital for normal CSF flow in the ventricles.[
The radiographic findings of BPC include retrocerebellar position of the cyst, morphologically normal vermis, cystic dilatation of the fourth ventricle, and some degree of compression of cerebellum.[
Endoscopic third ventriculostomy is an accepted form of treatment modality in symptomatic BPC.[
HISTORICAL EVOLUTION OF DESCRIPTION OF POSTERIOR FOSSA CYSTS
The literature provides us with fascinating insights into the evolution of various descriptive terminologies for posterior fossa anomalies. For instance, MCM was thought to be due to cerebellar atrophy and the term was used to describe any retrocerebellar CSF space with normal vermis and cerebellum.[
Cornips and colleagues[
Paladini et al.[
Studies by Blaicher et al.[
Vermis and Ventriculomegaly
Recently, there is increasing focus on accurate description of vermis when evaluating PF fluid collections in fetal and postnatal imaging. Moderately raised vermis can be due to vermian hypoplasia or DWM, or it could be due to persistent Blake pouch.[
The high level of persistent parental stress even with normal neurodevelopmental outcomes was a consistent finding in more than a third of cases in a study by Skreden et al.[
CONCLUSION
The inconsistencies in the classification of cerebellar and posterior fossa cystic anomalies are well documented in literature. Complex and protracted embryological development of cerebellum contributes to increased false positivity rate of many anomalies. There is increasing focus on accurate description of choroid plexus and vermis, especially in the setting of ventriculomegaly, based on current embryological data. In our case, like many other reported case series, there was inconsistent nomenclature with listing of broad differential diagnoses during prenatal period. There was adequate parental counseling with discussions on possible diagnoses and favorable and unfavorable developmental predictions. Even with retrospective evaluation, it could not be concluded whether this was a Blake’s pouch cyst or a retrocerebellar arachnoid cyst requiring emergent neurosurgical intervention.
Prenatal diagnosis, outcome prognostication, and parental counseling are fraught with difficulties. Information from fetal MRI is a valuable aid as there is a wide variation in the neurological prognoses based on nature of the anomaly. Better understanding of the nuances of normal brain development along with improved tissue resolution of fetal MRI is essential to translate this to patient care. Deriving at an accurate diagnosis has significant clinical relevance and may have an impact on decisions regarding the course of the pregnancy and postnatal neurodevelopmental outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ali ZS, Lang SS, Bakar D, Storm PB, Stein SC. Paediatric intracranial arachnoid cysts: Comparative effectiveness of surgical treatment options. Childs Nerv Syst. 2014. 30: 461-9
2. Barkovich AJ, Kjos BO, Norman D, Edwards MS. Revised classification of posterior fossa cysts and cyst like malformations based on the results of multiplanar MR imaging. AJR Am J Roentgenol. 1989. 153: 1289-300
3. Ber R, Bar-Yosef O, Hoffmann C, Shashar D, Achiron R, Katorza E. Normal fetal posterior fossa in MR imaging: New biometric data and possible clinical significance. AJNR Am J Neuroradiol. 2015. 36: 795-802
4. Bolduc ME, Limperopoulos C. Neurodevelopmental outcomes in children with cerebellar malformations: A systematic review. Dev Med Child Neurol. 2009. 51: 256-67
5. Boltshauser E. Cerebellum-small brain but large confusion: A review of selected cerebellar malformations and disruptions. Am J Med Genet A. 2004. 126A: 376-85
6. Blaicher W, Bernaschek G, Deutinger J, Schmidt AM, Schindler E, Prayer D. Fetal and early postnatal magnetic resonance imaging: Is there a difference?. J Perinat Med. 2004. 32: 53-7
7. Conte G, Parazzini C, Falanga G, Cesaretti C, Izzo G, Rustico M. Diagnostic value of prenatal MR imaging in the detection of brain malformations in fetuses before the 26th week of gestational age. AJNR Am J Neuroradiol. 2016. 37: 946-51
8. Cornips EM, Overvliet GM, Weber JW, Postma AA, Hoeberigs CM, Baldewijns MM. The clinical spectrum of Blake’s pouch cyst: Report of six illustrative cases. Childs Nerv Syst. 2010. 26: 1057-64
9. Doherty D, Millen KJ, Barkovich AJ. Midbrain and hindbrain malformations: Advances in clinical diagnosis, imaging, and genetics. Lancet Neurol. 2013. 12: 381-93
10. Gandolfi Colleoni G, Contro E, Carletti A, Ghi T, Campobasso G, Rembouskos G. Prenatal diagnosis and outcome of fetal posterior fossa fluid collections. Ultrasound Obstet Gynecol. 2012. 39: 625-31
11. Kau T, Marterer R, Kottke R, Birnbacher R, Gellen J, Nagy E. Blake’s pouch cysts and differential diagnoses in prenatal and postnatal MRI: A pictorial review. Clin Neuroradiol. 2020. 30: 435-45
12. Limperopoulos C, Robertson RL, Khwaja OS, Robson CD, Estroff JA, Barnewolt C. How accurately does current fetal imaging identify posterior fossa anomalies?. AJR Am J Roentgenol. 2008. 190: 1637-43
13. Limperopoulos C, Robertson RL, Estroff JA, Barnewolt C, Levine D, Bassan H. Diagnosis of inferior vermian hypoplasia by fetal magnetic resonance imaging: Potential pitfalls and neurodevelopmental outcome. Am J Obstet Gynecol. 2006. 194: 1070-6
14. Malinger G, Lev D, Lerman-Sagie T. The fetal cerebellum. Pitfalls in diagnosis and management. Prenat Diagn. 2009. 29: 372-80
15. Marin-Sanabria EA, Yamamoto H, Nagashima T, Kohmura E. Evaluation of the management of arachnoid cyst of the posterior fossa in pediatric population: Experience over 27 years. Childs Nerv Syst. 2007. 23: 535-42
16. Milani HJ, Barreto EQ, Ximenes RL, Baldo CA, Araujo E, Moron AF. Foetal posterior fossa malformations: Review of the current knowledge. Radiol Bras. 2019. 52: 380-6
17. Nelson MD, Maher K, Gilles FH. A different approach to cysts of the posterior fossa. Pediatr Radiol. 2004. 34: 720-32
18. O’Rahilly R, Müller F, Bossy J. Atlas of the stages of development of the external forms of the brain in the human embryo. Arch Anat Histol Embryol. 1986. 69: 3-39
19. Paladini D, Quarantelli M, Pastore G, Sorrentino M, Sglavo G, Nappi C. Abnormal or delayed development of the posterior membranous area of the brain: Anatomy, ultrasound diagnosis, natural history and outcome of Blake’s pouch cyst in the fetus. Ultrasound Obstet Gynecol. 2012. 39: 279-87
20. Parisi MA, Dobyns WB. Human malformations of the midbrain and hindbrain: Review and proposed classification scheme. Mol Genet Metab. 2003. 80: 36-53
21. Patel TR, Bannister CM, Thorne J. A study of prenatal ultrasound and postnatal magnetic imaging in the diagnosis of central nervous system abnormalities. Eur J Pediatr Surg. 2003. 13: S18-22
22. Poretti A, Meoded A, Rossi A, Raybaud C, Huisman TA. Diffusion tensor imaging and fiber tractography in brain malformations. Pediatr Radiol. 2013. 43: 28-54
23. Robinson AJ, Ederies MA. Diagnostic imaging of posterior fossa anomalies in the fetus. Semin Fetal Neonatal Med. 2016. 21: 312-20
24. Skreden M, Skari H, Malt UF, Haugen G, Pripp AH, Faugli A. Long-term parental psychological distress among parents of children with a malformation a prospective longitudinal study. Am J Med Genet A. 2010. 152A: 2193-202
25. Tarui T, Limperopoulos C, Sullivan NR, Robertson RL, du Plessis AJ. Long-term developmental outcome of children with a fetal diagnosis of isolated inferior vermian hypoplasia. Arch Dis Child Fetal Neonatal Ed. 2014. 99: F54-8
26. Tortori-Donati P, Fondelli MP, Rossi A, Carini S. Cystic malformations of the posterior cranial fossa originating from a defect of the posterior membranous area. Mega cisterna magna and persisting Blake’s pouch: Two separate entities. Childs Nerv Syst. 1996. 12: 303-8
27. Trompoukis P, Papantoniou N, Chlapoutaki C, Mesogitis S, Antsaklis A. Fetal MRI: Is it really helpful?. J Matern Fetal Neonatal Med. 2012. 25: 2363-8
28. Vakakmudi UB, Rangasami R, Gopinath VN. Prenatal Blake pouch cyst with hydrocephalus. Neurol India. 2016. 64: 830-1