- Departments of Neurosurgery, Shiga University of Medical Science, Otsu, Shiga, Japan.
- Clinical Laboratory Medicine and Division of Diagnostic Pathology, Shiga University of Medical Science, Otsu, Shiga, Japan.
Correspondence Address:
Naoki Nitta, Department of Neurosurgery, Shiga University of Medical Science, Otsu, Shiga, Japan.
DOI:10.25259/SNI_28_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naoki Nitta1, Suzuko Moritani2, Tadateru Fukami1, Kazuhiko Nozaki1. Characteristics of cranial vault lymphoma from a systematic review of the literature. 03-Jun-2022;13:231
How to cite this URL: Naoki Nitta1, Suzuko Moritani2, Tadateru Fukami1, Kazuhiko Nozaki1. Characteristics of cranial vault lymphoma from a systematic review of the literature. 03-Jun-2022;13:231. Available from: https://surgicalneurologyint.com/surgicalint-articles/11638/
Abstract
Background: Cranial vault lymphomas are rare and their clinical features are often similar to those of cranial vault meningiomas. The objective of this review was to identify the features helpful for differentiating lymphomas of the cranial vault, from meningiomas which were the most common diagnosis before the definitive pathological diagnosis.
Methods: The inclusion criterion was a histologically proven malignant lymphoma initially appearing in the calvarium. We conducted a literature search of the electronic PubMed and Ichushi-Web databases up to June 1, 2020. Cranial vault lymphoma that was diagnosed after an original diagnosis of lymphoma in a nodal or soft-tissue site was excluded from the study. Descriptive analyses were used to present the patient characteristics.
Results: A total of 111 patients were found in 98 eligible articles. Almost all studies were case reports. The most common symptom was a growing subcutaneous scalp mass (84%) present for a mean duration of 5.9 months before the patient presented for treatment in analyzable cases; this fast growth may distinguish lymphomas from meningiomas. The tumor vascularization was often inconspicuous or poor, unlike well-vascularized meningiomas. A disproportionately small amount of skull destruction compared with the soft-tissue mass was observed in two-thirds of the analyzable cases.
Conclusion: This qualitative systematic review identified several features of cranial vault lymphomas that may be useful in differentiating them from meningiomas, including a rapidly growing subcutaneous scalp mass, poor vascularization, and limited skull destruction relative to the size of the soft-tissue mass.
Keywords: Calvarial lymphoma, Calvarium, Lymphosarcoma, Reticulum cell sarcoma, Skull
INTRODUCTION
Malignant lymphoma of the bone is uncommon and, hence, presents diagnostic and therapeutic problems.[
MATERIALS AND METHODS
Eligibility criteria
The inclusion criterion was a histologically proven malignant lymphoma initially appearing in the calvarium. Cranial vault lymphoma that was diagnosed after an original diagnosis of lymphoma in a nodal or soft-tissue site was excluded from the study. Skull base lymphoma and dural lymphoma were also excluded from the study. Articles whose full text was unable to be located were excluded from the study. We excluded systematic and retrospective review articles and case series articles that did not include case-specific data.
Information sources and search strategy
We conducted a literature search of the electronic PubMed and Ichushi-Web databases up to June 1, 2020, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We extracted all human reports on cranial vault lymphomas, using the following terms: cranial vault lymphoma; skull lymphoma; calvarial lymphoma; transcalvarial lymphoma; dural lymphoma; and combinations of the variables of lymphoma, lymphosarcoma (an obsolete classification of non-Hodgkin lymphoma), reticulum cell sarcoma (another obsolete classification of non-Hodgkin lymphoma), cranial, vault, skull, and calvarium. The search resulted in 1492 PubMed citations and 391 Ichushi-Web citations, 113 of which were duplicated, resulting in a total of 1770 articles [
Selection process
Two reviewers (N.N. and T.F.) independently and in duplicate screened, reviewed, and discussed all the selected articles. The full text of all eligible articles was reviewed, and their data extracted and collated. In cases where questions regarding the inclusion of certain articles arose, this was discussed with a third reviewer, K.N.
Data collection process and data items
Data of the eligible works were obtained through careful analysis of the full text by two authors (N.N. and T.F) independently. The senior author, K.N., was available in case of a split decision. Questions arising as to pathological diagnosis were discussed with a pathologist, S.M. We analyzed the clinical and radiological characteristics of the patients, as well as their treatments and survival in these published studies. Specifically, we extracted the following items: age of the patient; sex; clinical symptoms; location of tumors; findings of palpation; skin condition; speed of growth; existence of other lesions; types of treatment; extent of resection; duration of follow-up and outcomes; laboratory data; imaging data of skull X-ray, angiogram, computed tomography (CT), magnetic resonance imaging (MRI), and others; bone images on CT; extra- and intracranial tumor extension on CT and MRI; dural tail and brain invasion on MRI; and histopathological types. Due to the heterogeneity of patient descriptions, some clinical and imaging features were not explicitly reported for each patient. We extracted and reported only unambiguously described data. Data on clinical and imaging features were also extracted from the patient imaging data. Evaluation of publication bias was not feasible because of heterogeneity and because most of the included studies were case reports and case series.
Statistical analysis
Descriptive analyses were used to present the patient characteristics. Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as number and percentage. Because of the limited follow-up data included in each intervention and the lack of standardization of assays and treatments across the many laboratories included in the present review, we were unable to statistically compare the findings on images and the changes in clinical outcomes. All calculations were performed with JMP 13.2.1 (SAS Institute, Inc., Cary, North Carolina, U.S.).
RESULTS
From among the articles found, we selected all studies reporting patients with cranial vault lymphoma (n = 106) without limitation of language and identified additional studies from the reference lists of the articles (n = 3). After discarding duplicate references and publications (n = 2), as well as excluding reports of secondary cranial vault lymphoma, skull base lymphoma, or dural lymphoma (n = 9), we settled on 98 articles for careful review. The number of articles retained at each stage of data acquisition is shown in a PRISMA flowchart [
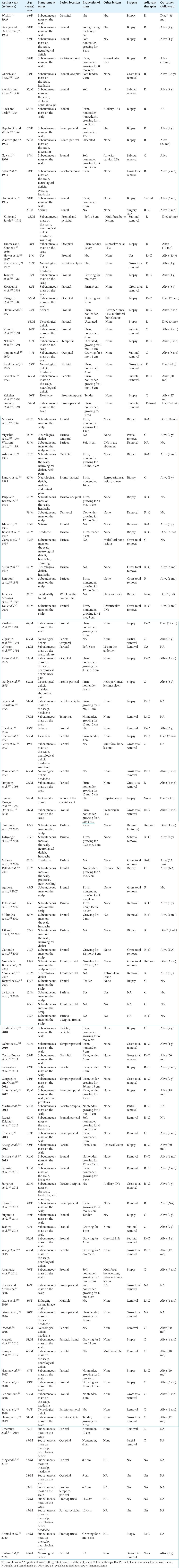
We found 111 cases of histologically proven malignant lymphomas initially appearing in the cranial vault in the 98 articles and analyzed the data of 111 patients [
The average patient age was 52 ± 20 years (range, 3–85 years) [
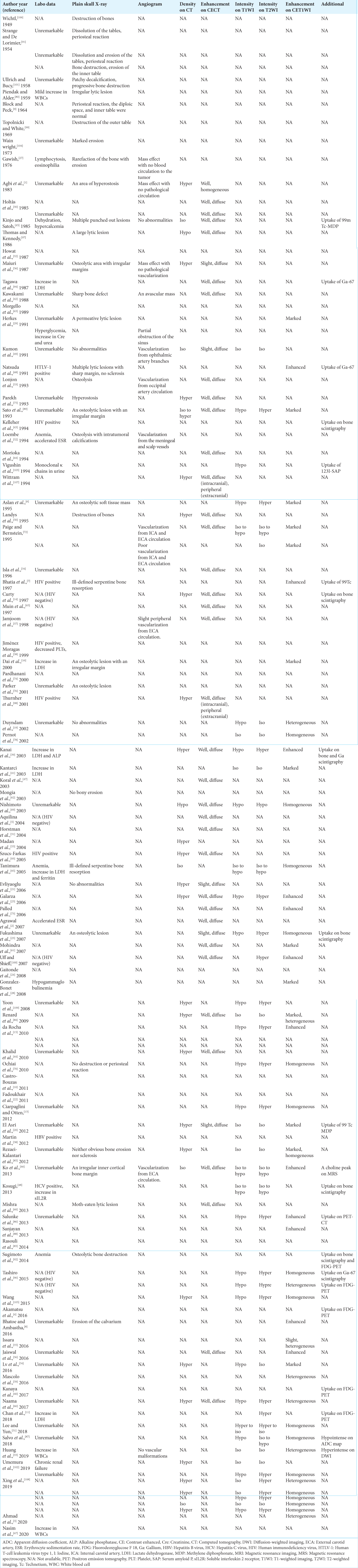
Laboratory data were described in 59 cases, with human immunodeficiency virus positivity in 5 cases (8%), elevated lactate dehydrogenase in 6 cases (10%), anemia in 4 cases (7%), increased white blood cell count in 3 cases (5%), elevated erythrocyte sedimentation rate in 2 cases (3%), elevated alkaline phosphatase in 1 case (2%), increased soluble interleukin-2 receptor (sIL2R) in 1 case (2%), and decreased platelets in 1 case (2%) [Table S1]. Findings on plain skull X-ray were described in 38 cases, including osteolytic changes at the tumor site in 29 cases (76%), no change in 6 cases (16%), hyperostosis in 2 cases (5%), and periosteal reaction in 4 cases (11%). A sharp margin of the skull lesion was observed in 6 cases (16%) and an indistinct or irregular margin of the skull lesion in 15 cases (40%). In 12 cases with description of tumor vascularization on angiogram, no or poor tumor vascularization was found in 7 cases (58%). If any vascularization was present, it was mainly derived from the external carotid artery circulation. Among 32 cases with analyzable CT data, the tumor was hyperdense in 25 cases (78%), isodense in 5 cases (16%), and hypodense in 2 cases (6%). Among 42 cases with contrast-enhanced (CE) CT data, the tumor was enhanced well in 37 cases (88%) and slightly in 5 cases (12%). Most tumors were enhanced diffusely, either homogeneously or heterogeneously, whereas some extracranial components showed peripheral enhancement. On MRI, T1-weighted imaging (T1WI) with analyzable data (n = 38) showed a hyper- and isointense tumor in 1 case (3%), isointense tumor in 11 cases (29%), iso- and hypointense tumor in 4 cases (11%), and hypointense tumor in 22 cases (58%). T2-weighted imaging (T2WI) (n = 40) showed a hyperintense tumor in 21 cases (53%), hyper- and isointense tumor in 1 case (3%), isointense tumor in 12 cases (30%), iso- and hypointense tumor in 5 cases (13%), and hypointense tumor in 1 case (3%). CE-T1WI (n = 48) showed an enhancing tumor in 48 cases (100%). The tumors tended to show uptake on 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) and on bone scintigraphy and gallium scintigraphy, which were also used for the evaluation of lesions outside the cranial vault.
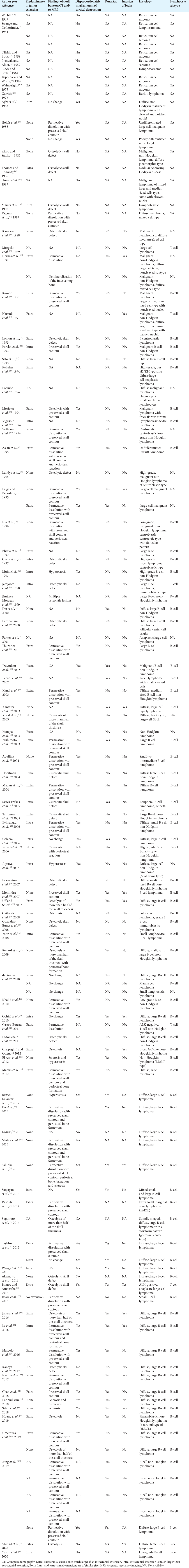
Extra- and intracranial extensions of the tumor were evaluated by CT, MRI, or both in 87 cases [Table S2]; the extracranial extension was much larger than the intracranial extension in 35 cases (40%), both were nearly the same size in 35 cases (40%), the intracranial extension was much larger than the extracranial extension in 16 cases (18%), and there was neither intra- nor extracranial extension in 1 case (1%). Bone changes of the cranial vault due to the tumor were evaluated by CT, MRI, or both in 84 cases. The images showed no skull changes and/or preserved skull contour in 11 cases (13%), osteolytic changes in 68 cases (81%), hyperostosis in 4 cases (5%), and only sclerosis at the lesion in 1 case (1%). In the 68 cases with osteolytic skull changes due to the tumor, the skull was penetrated or dissolved to less than half of the thickness in 26 cases (38%), whereas permeative dissolution with relatively preserved skull contour was observed in 35 cases (51%). Periosteal bone formation was observed in 10 cases (15%). In seven cases, there was no detailed description. A disproportionately small area of cortical destruction of the cranial vault relative to the volume of the extra- or intracranial soft-tissue mass, which we defined as cortical destruction less than one-fifth of the soft-tissue mass in diameter, was observed in 50 cases (67%) on CT, MRI, or both (n = 75). On MRI (n = 57), a dural tail was observed in 42 cases (74%), and invasion of the brain was observed in 15 cases (26%).
Surgery was detailed in 98 cases, of which 45 (46%) involved a biopsy or partial removal and 53 (54%) involved subtotal or gross total removal [
The type of lymphocyte was described in 80 cases: 75 (94%) were B-cell lymphomas and 5 (6%) were T-cell lymphomas [Table S2]. Diffuse large B-cell lymphoma (DLBCL) was the most common, being reported in 34 cases.
To determine the rates of survival, we excluded cases in which the patients died of a cause unrelated to the skull lesion and included cases with follow-up periods of more than 6 months (n = 74) or 1 year (n = 54). In the included cases, 67 (90.5%) and 45 (83.3%) patients were alive at the follow-up of 6 months and 1 year, respectively [
DISCUSSION
We reviewed the demographic, clinical, and imaging characteristics of cranial vault lymphoma to specify the features that might be helpful for differential diagnosis, especially between lymphomas and meningiomas of the cranial vault.
When a tumor with intra- and extracranial extension sandwiching the skull is seen, meningioma with extracranial extension is often first suspected. In many cases, the subcutaneous scalp mass was firm and nontender, which is also similar to meningioma with extracranial extension. However, in our review, the subcutaneous scalp mass grew very rapidly before the patient presented for treatment, for a mean duration of 5.9 months, which is atypical for meningioma, which is generally slow growing.[
Laboratory data were unremarkable in many cases. Kosugi et al.[
The extracranial component tended to be at least as large as the intracranial component in cranial vault lymphoma. Because meningioma usually originates from the meninges, theoretically, it tends to grow intracranially rather than extracranially. Extracranial-dominant extension might, therefore, also contribute to distinguishing cranial vault lymphoma from meningioma.
However, other findings are unlikely to clarify the diagnosis. For example, the tumors tended to be hyper- to isodense on CT, iso- to hypointense on T1WI, and hyper-to isointense on T2WI, which are nonspecific features of skull tumors. Although a dural tail was observed in many cases, as reported by Xing et al.,[
It has been suggested that lymphoma cells infiltrate the spaces within the diploe and extend along the emissary veins to infiltrate the soft tissues on either side of the bone.[
After histological confirmation of the diagnosis, patients were usually treated with adjuvant chemotherapy, radiotherapy, or both. Although the best treatment for cranial vault lymphoma has not been elucidated because of the paucity of cases and lack of clinical studies, treatments based on those for systemic malignant lymphoma tended to be adopted.
B-cell lymphomas accounted for approximately 94% of the cranial vault lymphomas, whereas T-cell lymphomas roughly accounted for the remaining 6% of cases. Peripheral T-cell lymphomas account for 6–10% of all cases of non-Hodgkin lymphoma[
In analyzable follow-up data, 67 and 45 patients were alive after 6 months (n = 74) and 1 year (n = 54), respectively [
In the present review, we have provided objective data from patients reported in published studies. The difficulty in systematically reviewing the data reported in the literature is the heterogeneity of the data. In particular, almost all reports reviewed for this study were case reports. Because of the limited number and the heterogeneity of described data of preoperative findings and treatments, we could perform only descriptive analyses. Another weakness of the present review resulted from biased reporting in the published studies, with likely underreporting of recent cases in the United States and European countries because of decreased novelty in inverse proportion to the accumulation of reported cases. The data that were more likely to be reported, if present, included findings on MRI and CT. Findings of plain skull X-ray and angiography and long-term follow-up data were less likely to be reported.
The strength of the present review is the comprehensive nature of the literature search. We analyzed cases from all over the world, reported not only in English but also in four non-English languages.
CONCLUSION
Cranial vault lymphoma is a rare entity among skull tumors. To the best of our knowledge, this is the largest reported pooled database describing cranial vault lymphoma patients. The most common symptom was a rapidly growing subcutaneous scalp mass. The tumor was poorly vascularized on angiography. Skull destruction on images was mild and disproportionately small despite the large size of the extracranial and/or intracranial component in two-thirds of the cases. These features should help to distinguish lymphoma from meningioma.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY TABLES
Acknowledgments
The authors thank ELSS, Inc. (https://www.elss.co.jp/ ) for English-language editing of this manuscript.
References
1. Agbi CB, Bannister CM, Turnbull IW. Primary cranial vault lymphoma mimicking a meningioma. Neurochirurgia (Stuttg). 1983. 26: 130-2
2. Agrawal A, Makannavar JH, Shetty JP, Shetty RK, Shetty L. Frontal convexity primary lymphoma masquerading meningioma: A case report and review of literature. Indian J Cancer. 2007. 44: 36-7
3. Ahmad W, Hassan A, Niazi IK. Cranial vault lymphoma-an unusual presentation of primary disease. J Pak Med Assoc. 2020. 70: 767-8
4. Akamatsu Y, Kanamori M, Uenohara H, Tominaga T. Bone regeneration after chemotherapy for vault lymphoma. BMJ Case Rep. 2016. 2016: bcr2015213524
5. Aquilina K, O’Brien DF, Phillips JP. Diffuse primary nonHodgkin’s lymphoma of the cranial vault. Br J Neurosurg. 2004. 18: 518-23
6. Aslan Y, Okten A, Demirci A. Primary Burkitt’s lymphoma of the cranial vault in a child. Pediatr Radiol. 1995. 25: S232-3
7. Bhatia S, Smally AJ, Dekker P. Primary non-Hodgkin’s lymphoma of the cranial vault. Clin Oncol (R Coll Radiol). 1997. 9: 195-6
8. Bhatoe HS, Ambastha R. Primary non Hodgkin’s lymphoma of the cranial vault in a child. J Neurooncol. 2016. 126: 209-11
9. Block A, Peck HM. Radiological notes: Lymphosarcoma of the scalp with bony invasion. J Mt Sinai Hosp N Y. 1964. 31: 241-4
10. Castro-Bouzas D, Prieto-González Á, Serramito-García R, Santín-Amo JM, Reyes-Santías RM, Allut AG. Primary cranial vault lymphoma. Rev Neurol. 2011. 53: 735-8
11. Chan DY, Chan DT, Poon WS, Wong GK. Primary cranial vault lymphoma. Br J Neurosurg. 2018. 32: 214-5
12. Chisholm KM, Ohgami RS, Tan B, Hasserjian RP, Weinberg OK. Primary lymphoma of bone in the pediatric and young adult population. Hum Pathol. 2017. 60: 1-10
13. Ciarpaglini R, Otten P. Primary cranial vault lymphoma with brain infiltration: Case report and review of the literature. Br J Neurosurg. 2012. 26: 756-8
14. Curty B, Kernan J, Favre J. Malignant non-Hodgkin’s lymphoma of the cranial vault: A case report. Br J Neurosurg. 1997. 11: 433-6
15. da Rocha AJ, da Rocha TM, da Silva CJ, Paes RP, Bruniera P, Chiattone CS. Cranial vault lymphoma: A systematic review of five patients. J Neurooncol. 2010. 100: 9-15
16. Dai MS, Ho CL, Chen CY, Chen TM, Yu CP, Chao TY. Lymphoma of bone with initial presentation as a calvarial mass. Ann Hematol. 2000. 79: 700-2
17. Deckert MP, Kluin PM, Ferry JA, Louis DN, Wiestler OD, Cavenee WK.editors. Lymphomas. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Researcher on Cancer; 2016. p.
18. Djindjian M, Raulo Y, Gaston A, Decq P, Gabriel-Cordonnier E, Poirier J. Intra-extracranial meningioma in the cranial vault. Neurochirurgie. 1995. 41: 329-36
19. Duyndam DA, Biesma DH, van Heesewijk JP. Primary nonHodgkin’s lymphoma of the cranial vault; MRI features before and after treatment. Clin Radiol. 2002. 57: 948-50
20. El Asri AC, Akhaddar A, Baallal H, Boulahroud O, Mandour C, Chahdi H. Primary lymphoma of the cranial vault: Case report and a systematic review of the literature. Acta Neurochir (Wien). 2012. 154: 257-65
21. Evliyaoğlu C, Ilbay K, Ercin C, Ceylan S. Primary cranial vault lymphoma presenting as a traumatic subdural hematoma. Zentralbl Neurochir. 2006. 67: 26-9
22. Fadoukhair Z, Lalya I, Amzerin M, Elkhanoussi B, Sbitti Y, Boutayeb S. Successful management of primary non Hodgkins lymphoma of the cranial vault. Pan Afr Med J. 2011. 8: 50
23. Fukushima Y, Oka H, Utsuki S, Nakahara K, Fujii K. Primary malignant lymphoma of the cranial vault. Acta Neurochir (Wien). 2007. 149: 601-4
24. Gaitonde S, Patel R, Alagiozian-Angelova V, Kadkol S, Peace D. Primary low grade follicular lymphoma of cranial vault mimicking lipoma at presentation. Acta Oncol. 2008. 47: 326-9
25. Galarza M, Gazzeri R, Elfeky HA, Johnson RR. Primary diffuse large B-cell lymphoma of the dura mater and cranial vault. Case report and literature review. Neurosurg Focus. 2006. 21: E10
26. Galldiks N, Albert NL, Sommerauer M, Grosu AL, Ganswindt U, Law I. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017. 19: 1576-87
27. Gawish HH. Primary Burkitt’s lymphoma of the frontal bone. Case report. J Neurosurg. 1976. 45: 712-5
28. González-Bonet LG, Gutiérrez-Herrera JM, Gallego JM, Barcia JA. Primary immunoblastic B-cell lymphoma of the cranial vault. Acta Neurochir (Wien). 2008. 150: 507-8
29. Herkes GK, Partington MD, O’Neill BP. Neurological features of cranial vault lymphomas: Report of two cases. Neurosurgery. 1991. 29: 898-901
30. Holtås S, Monajati A, Utz R. Computed tomography of malignant lymphoma involving the skull. J Comput Assist Tomogr. 1985. 9: 725-7
31. Horstman AL, Go RS, Bottner WA. Cranial vault lymphoma. Haematologica. 2004. 89: EIM06
32. Howat AJ, Thomas H, Waters KD, Campbell PE. Malignant lymphoma of bone in children. Cancer. 1987. 59: 335-9
33. Huang J, Tang D, Xu Y, Wang X, Yu C, Dong Y. Head trauma complicated with primary cranial vault lymphoma: A case report. Medicine (Baltimore). 2019. 98: e14465
34. Isla A, Alvarez F, Gutiérrez M, Gamallo C, García-Blázquez M, Vega A. Primary cranial vault lymphoma mimicking meningioma. Neuroradiology. 1996. 38: 211-3
35. Issara K, Yossi S, Caraivan I. Primary lymphoma of the skull: Case report and literature review. Cancer Radiother. 2016. 20: 811-4
36. Jaiswal M, Gandhi A, Purohit D, Singhvi S, Mittal RS. Primary non-Hodgkin’s lymphoma of the skull with extra and intracranial extension presenting with bulky scalp mass lesion. Asian J Neurosurg. 2016. 11: 444
37. Jamjoom AB, Jamjoom ZA, Naim-Ur-Rahman Cheema MA. Primary midline cranial vault lymphoma simulating a parasagittal meningioma: The role of angiography in preoperative diagnosis. Neurosurg Rev. 1998. 21: 202-5
38. Jiménez Moragas JM, Sánchez Rodríguez A, Sierra Camerino R, Palomo González MJ. Non-Hodgkin’s disease of the skull in a patient with AIDS. An Med Interna. 1999. 16: 186-8
39. Kanai M, Kawano K, Murakami T, Saitou M, Kikumoto N. A case of malignant lymphoma of the cranial vault. No Shinkei Geka. 2003. 31: 419-24
40. Kanaya M, Endo T, Hashimoto D, Endo S, Takemura R, Okada K. Diffuse large B-cell lymphoma with a bulky mass in the cranial vault. Int J Hematol. 2017. 106: 147-8
41. Kantarci M, Erdem T, Alper F, Gundogdu C, Okur A, Aktas A. Imaging characteristics of diffuse primary cutaneous B-cell lymphoma of the cranial vault with orbital and brain invasion. AJNR Am J Neuroradiol. 2003. 24: 1324-6
42. Kawakami KK, Inagaki T, Numa Y, Kawamura Y, Matsumura H. Two cases of malignant lymphoma in the skull. Prog CT. 1988. 10: 217-21
43. Kelleher AD, Brew BJ, Milliken ST. Intractable headache as the presenting complaint of AIDS-related lymphoma confined to bone. J Acquir Immune Defic Syndr (1988). 1994. 7: 629-30
44. Khalid M, Siddiqui MA, Qaseem SM, Ahmad M, Khalid S. Multifocal primary lymphoma of the cranial vault in a nonimmunocompromised adolescent. JBR-BTR. 2010. 93: 296-8
45. Kinjo T, Satoh T. A case of malignant lymphoma in the skull after head injury associated with multiple bone tumors. No Shinkei Geka. 1985. 13: 1191-6
46. Ko MJ, Hwang SN, Park YS, Nam TK. Primary malignant lymphoma of the cranial vault with extra-and intracranial extension. Brain Tumor Res Treat. 2013. 1: 32-5
47. Koral K, Curran JG, Thompson A. Primary non-Hodgkin’s lymphoma of the temporal bone. CT findings. Clin Imaging. 2003. 27: 386-8
48. Kosugi S, Kume M, Sato J, Sakuma I, Moroi J, Izumi K. Diffuse large B-cell lymphoma with mass lesions of skull vault and ileocecum. J Clin Exp Hematop. 2013. 53: 215-9
49. Kumon Y, Sakaki S, Nakano K, Fukui K, Kohno H, Kurihara K. Primary malignant lymphoma of the skull presenting as a growing mass in the forehead; a case report. No Shinkei Geka. 1991. 19: 279-83
50. Landys KE, Berg GE, Torgerson JS, Klingenstierna HA, Ridell BS, Johansson BR. Bulky centroblastic non-Hodgkin’s lymphoma of the cranium vault mimicking brain involvement managed with chemotherapy: A case report. Cancer. 1995. 76: 1261-7
51. Lee SH, Yun SJ. Early stage primary cranial vault lymphoma in a 50-year-old man: Presenting as only sclerosis and mimicking osteoma. Ann Hematol. 2018. 97: 183-4
52. Loembe PM, Minko-Mi-Etoua D, Mekemeza-M’Obiang L, Assengone-Zeh Y, Mwanyombet-Ompounga L. Primary lymphoma of frontoparietal localization in the cranial vault. Report of a case. Neurochirurgie. 1994. 40: 369-71
53. Lonjon M, Hofman P, Thyss A, Paquis P, Roche JL. Primary lymphoma of the cranial vault. Apropos of a case. Neurochirurgie. 1993. 39: 50-3
54. Lv ZW, Cheng KL, Tian HJ, Han XM. Primary diffuse large B-cell lymphoma of the dura with skull and scalp involvement: A case report and brief review of the literature. Oncol Lett. 2016. 11: 3583-8
55. Madan RN, Narula MK, Anand R, Gurtoo A. Primary cutaneous non-Hodgkin lymphoma of the scalp: A case report. Indian J Radiol Imaging. 2004. 14: 385-387
56. Maiuri F, Corriero G, Giamundo A. Primary lymphoma of the cranial vault. J Neurosurg Sci. 1987. 31: 183-6
57. Marchi E, O’Connor OA. The rapidly changing landscape in mature T-cell lymphoma (MTCL) biology and management. CA Cancer J Clin. 2020. 70: 47-70
58. Martin J, Ramesh A, Kamaludeen M, Udhaya Ganesh K, Martin JJ. Primary non-hodgkin’s lymphoma of the scalp and cranial vault. Case Rep Neurol Med. 2012. 2012: 616813
59. Mascolo M, Piccolo V, Iannuzzo G, Di Lorenzo P, De Rosa G, Staibano S. Primary cutaneous diffuse large B-cell lymphoma with cranial vault and dura mater involvement. J Eur Acad Dermatol Venereol. 2016. 30: 186-7
60. Mishra SS, Senapati SB, Dhir MK, Das S, Burma S. A case of primary skull lymphoma: Review of literature. Neurol India. 2013. 61: 334-6
61. Mohindra S, Gupta R, Sharma T, Das A, Radotra BD. Calvarial lymphoma. Br J Neurosurg. 2007. 21: 629-30
62. Mongia S, Shukla D, Devi BI, Reddy TV. Primary cranial vault non-Hodgkin’s lymphoma. Neurol India. 2003. 51: 293-4
63. Morgello S, Maiese K, Petito CK. T-cell lymphoma in the HIV: Clinical and pathologic features. Neurology. 1989. 39: 1190-6
64. Morioka T, Tashima T, Nishio S, Nishie E, Fukui M, Okamura T. Malignant lymphoma of the scalp at the site of a previous blunt trauma: Report of two cases. Surg Neurol. 1994. 42: 117-20
65. Muin IA, Saffari HM, Hasimah YN. Primary non-Hodgkin’s lymphoma of the cranial vault mimicking a meningioma: A case report. Med J Malaysia. 1997. 52: 86-8
66. Naama O, Gazzaz M, Boucetta M, Elmoustarchid B. Primary non-Hodgkin lymphoma of the cranial vault. Rev Neurol (Paris). 2017. 173: 668-70
67. Nasim MM, Chalif DJ, Demopoulos AM, Brody J, Lee-Huang R, Spitzer SG. Primary low-grade B-cell lymphoma of skull with translocation between immunoglobulin and interferon regulatory factor 4 genes. Int J Surg Pathol. 2020. 28: 330-5
68. Natsuda H, Muraki Y, Kobayashi T, Kozima H, Shibuya A, Nagasawa T. Adult T cell lymphoma/leukemia with alopecia, huge tumors of scalp and wide destruction of cerebral bone. Rinsho Ketsueki. 1991. 32: 537-41
69. Nishimoto T, Yuki K, Sasaki T, Imada Y, Murakami T, Kodama Y. A case of subcutaneous malignant lymphoma with Dura mater lesion. No Shinkei Geka. 2003. 31: 43-7
70. Ochiai H, Kawano H, Miyaoka R, Kawano N, Shimao Y, Kawasaki K. Primary diffuse large B-cell lymphomas of the temporoparietal Dura mater and scalp without intervening skull bone invasion. Neurol Med Chir (Tokyo). 2010. 50: 595-8
71. Ostrowski ML, Unni KK, Banks PM, Shives TC, Evans RG, O’Connell MJ. Malignant lymphoma of bone. Cancer. 1986. 58: 2646-55
72. Paige ML, Bernstein JR. Transcalvarial primary lymphoma of bone. A report of two cases. Neuroradiology. 1995. 37: 456-8
73. Palled S, Bedre G, Muckaden MA, Ramadwar M, Bhagwat R, Banavali S. Primary Burkitt’s lymphoma of frontal bone: A rare presentation. J Clin Oncol. 2006. 24: 4521-2
74. Pardhanani G, Ashkan K, Mendoza N. Primary non-Hodgkin’s lymphoma of the cranial vault presenting with unilateral proptosis. Acta Neurochir (Wien). 2000. 142: 597-8
75. Parekh HC, Sharma RR, Keogh AJ, Prabhu SS. Primary malignant non-Hodgkin’s lymphoma of cranial vault: A case report. Surg Neurol. 1993. 39: 286-9
76. Parker JR, López-Terrada D, Gresik MV, Vogel H, Baumgartner JE, Finegold MJ. Neutrophil-rich anaplastic large cell lymphoma of the skull presenting after head trauma. Pediatr Dev Pathol. 2001. 4: 397-401
77. Pear BL. Skeletal manifestations of the lymphomas and leukemias. Semin Roentgenol. 1974. 9: 229-40
78. Pernot P, Saint-Blancard P, Dulou R, Blondet E, Goasguen O. Primary dural lymphoma. A case report. Neurochirurgie. 2002. 48: 124-7
79. Perry AL, Budka H, von Deimling A, Sahm F, Rushing EJ, Mawrin C, Louis DN, Wiestler OD, Cavenee WK.editors. Meningioma. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Researcher on Cancer; 2016. p.
80. Piendak JS, Alder JW. Primary reticulum cell sarcoma of the skull with metastasis: Report of a case. Del Med J. 1959. 31: 306-9
81. Pui CH, Ip SH, Kung P, Dodge RK, Berard CW, Crist WM. High serum interleukin-2 receptor levels are related to advanced disease and a poor outcome in childhood nonHodgkin’s lymphoma. Blood. 1987. 70: 624-8
82. Ramadan KM, Shenkier T, Sehn LH, Gascoyne RD, Connors JM. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: A population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann Oncol. 2007. 18: 129-35
83. Rasouli J, Patel SA, Skovrlj B, Gologorsky Y, Mascitelli J, Petersen B. Primary extranodal marginal zone lymphoma involving the skull. J Clin Neurosci. 2014. 21: 351-3
84. Renard D, Campello C, Beraru O, Bouillot P, Labauge P. Teaching neuroimages: Primary diffuse large B-cell lymphoma of the cranial vault. Neurology. 2009. 73: e84-5
85. Rezaei-Kalantari K, Samimi K, Jafari M, Karimi MA, Ansari K, Davoodi M. Primary diffuse large B cell lymphoma of the cranial vault. Iran J Radiol. 2012. 9: 88-92
86. Salunke P, Garg R, Bal A, Kedia S, Bindal S. Primary malignant non-Hodgkin’s lymphoma of the skull vault in an immunocompetent patient. Neurol India. 2013. 61: 201-4
87. Salvo V, Brogna B, Sampirisi L, Casinelli A, Emanuela R. Diffuse-primary-B-cell lymphoma of the cranial vault presenting as stroke. Radiol Case Rep. 2018. 13: 658-62
88. Sanjayan R, Prabhakaran P, Surendran A, Narayanan G. Non-Hodgkin lymphoma of the cranial vault. Am J Med. 2013. 126: e7-8
89. Sato M, Saito T, Yamaguchi K. Primary malignant lymphoma of the skull presenting a huge mass lesion: Case report. No Shinkei Geka. 1993. 21: 1061-4
90. St Clair EG, McCutcheon IE.editors. Skull tumors. Youmans Neurological Surgery. Philadelphia, PA: Saunders; 2011. p.
91. Strange VM, De Lorimier AA. Reticulum cell sarcoma primary in the skull; report of three cases. Am J Roentgenol Radium Ther Nucl Med. 1954. 71: 40-50
92. Sugimoto KJ, Shimada A, Ichikawa K, Wakabayashi M, Sekiguchi Y, Izumi H. Primary platelet-derived growth factor-producing, spindle-shaped diffuse large B-cell lymphoma of the skull: A case report and literature review. Int J Clin Exp Pathol. 2014. 7: 4381-90
93. Szucs-Farkas Z, Peltzer J, Berger D, Braunschweig M. Aggressive lymphoma of the skull in a patient with AIDS. JBRBTR. 2005. 88: 152-3
94. Tagawa M, Momita S, Irie J. Primary B cell lymphoma of the skull following head trauma; a case report. Rinsho Ketsueki. 1987. 28: 589-93
95. Tanimura A, Adachi Y, Tanda M, Yuasa H, Ishii Y, Katou Y. Primary peripheral B cell lymphoma, Burkitt-like, of the cranial vault. Acta Haematol. 2005. 113: 258-61
96. Tashiro R, Kanamori M, Suzuki H, Utsunomiya A, Meguro K, Uenohara H. Diffuse large B cell lymphoma of the cranial vault: Two case reports. Brain Tumor Pathol. 2015. 32: 275-80
97. Thomas CV, Kennedy BJ. Primary Hodgkin’s disease of the skull following a 3-year history of pseudotumor cerebri. Cancer. 1986. 58: 318-20
98. Thurnher MM, Rieger A, Kleibl-Popov C, Schindler E. Malignant lymphoma of the cranial vault in an HIV-positive patient: Imaging findings. Eur Radiol. 2001. 11: 1506-9
99. Topolnicki W, White RJ. Primary reticulum cell sarcoma of the skull. Response to irradiation. Cancer. 1969. 24: 569-73
100. Uff CE, Shieff CL. Neurological picture. Massive transcalvarial lymphoma. J Neurol Neurosurg Psychiatry. 2007. 78: 769
101. Ullrich DP, Bucy PC. Primary reticulum cell sarcoma of the skull. Am J Roentgenol Radium Ther Nucl Med. 1958. 79: 653-7
102. Umemura T, Nakano Y, Soejima Y, Saito T, Kitagawa T, Miyaoka R. Characteristics of bone destruction in cranial vault lymphoma compared with other skull tumors. J UOEH. 2019. 41: 335-42
103. Vigushin DM, Hawkins PN, Hsuan JJ, Totty NF, Pepys MB. AL kappa amyloid in a solitary extradural lymphoma. J Neurol Neurosurg Psychiatry. 1994. 57: 751-4
104. Wainwright RW. Primary reticulum cell sarcoma of the skull. Med J Aust. 1973. 1: 499-500
105. Wang L, Ouayang T, Zhang N, Song Z, Gao J, Li X. Primary diffuse large B-cell dural lymphoma with bone and subcutaneous tissue involvement mimicking meningioma. J Craniofac Surg. 2015. 26: e492-4
106. Wichtl O. Reticulum cell sarcoma of the skull roof healed by X-rays. Wien Med Wochenschr. 1949. 99: 12
107. Wittram C, Nixon TE, Mackenzie JM. Non-Hodgkin’s lymphoma of the skull vault. Eur J Radiol. 1994. 19: 7-9
108. Xing Z, Huang H, Xiao Z, Yang X, Lin Y, Cao D. CT, conventional, and functional MRI features of skull lymphoma: A series of eight cases in a single institution. Skeletal Radiol. 2019. 48: 897-905
109. Yoon SH, Paek SH, Park SH, Kim DG, Jung HW. Non-Hodgkin lymphoma of the cranial vault with retrobulbar metastasis mimicking a subacute subdural hematoma: Case report. J Neurosurg. 2008. 108: 1018-20