- Department of Neurology and Neurosurgery, Stavropol State Medical University, Stavropol, Russian Federation
- Clinic of Neurosurgery, N. V. Sklifosovsky Research Institute for Emergency Medicine, Moscow Healthcare Department, Moscow, Russian Federation
- Department of General Medicine, Stavropol State Medical University, Stavropol, Russian Federation
- Laboratory of Biochemistry, Stavropol Research Institute for Plague Control, Stavropol, Russian Federation.
Correspondence Address:
Michael Lebenstein-Gumovski, Department of Neurology and Neurosurgery, Stavropol State Medical University, Stavropol, Russian Federation.
DOI:10.25259/SNI_927_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael Lebenstein-Gumovski1, Tanzila Rasueva2, Alexander Zharchenko3, Robert Bashahanov3, Dmitry A. Kovalev4, Andrey Zhirov4, Anton Shatokhin1, Andrey Grin2. Chitosan/PEG-mediated spinal cord fusion after complete dorsal transection in rabbits – functional results at 30 days. 13-Dec-2023;14:423
How to cite this URL: Michael Lebenstein-Gumovski1, Tanzila Rasueva2, Alexander Zharchenko3, Robert Bashahanov3, Dmitry A. Kovalev4, Andrey Zhirov4, Anton Shatokhin1, Andrey Grin2. Chitosan/PEG-mediated spinal cord fusion after complete dorsal transection in rabbits – functional results at 30 days. 13-Dec-2023;14:423. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12665
Abstract
Background: The aim was to study functional recovery in experimental animals (rabbits) with transected spinal cords treated with a combination of photo-cross-linked chitosan in a homogeneous mixture with polyethylene glycol (PEG-chitosan).
Methods: 20 rabbits (n = 10 experimental and n = 10 controls) were submitted to complete spinal cord transection at T9. The experimental group received an intraoperative injection of PEG-chitosan. Neurological recovery was assessed using the modified Basso, Beattie, and Bresnahan scale.
Results: In the experimental group, partial recovery of movements, sensory function, and sphincter control were all observed by postoperative day 30. Paraplegia and anesthesia persisted in the control group; 4 controls died versus none in the test group.
Conclusion: PEG-chitosan is a candidate for neurological restoration after spinal paralysis.
Keywords: Chitosan, NeuroPEG, PEG, PEG-chitosan, Polyethylene glycol, Spinal cord injury, Spinal injury
INTRODUCTION
Since 2013, fusogens such as polyethylene glycol (PEG) have been tested in animal models of complete spinal cord transection with almost full recovery of ambulation.[
Here, we test a combination of PEG and chitosan[
MATERIALS AND METHODS
The study protocol was approved by the Local Ethics Committee of the Stavropol State Medical University (March 12, 2020). This trial was carried out in accordance with the ethical standards of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes.
We used chitosan with a molecular weight of 15 kDa (Merck, Germany), stabilized by photo-cross-linking, in a homogeneous mixture with PEG with a molecular weight of 600 kDa (PEG-600, Merck, Germany) at a concentration of 20 mg/mL (PEG-chitosan). The resulting mixture was sterilized and stored in sealed tubes at a temperature of 4°C.
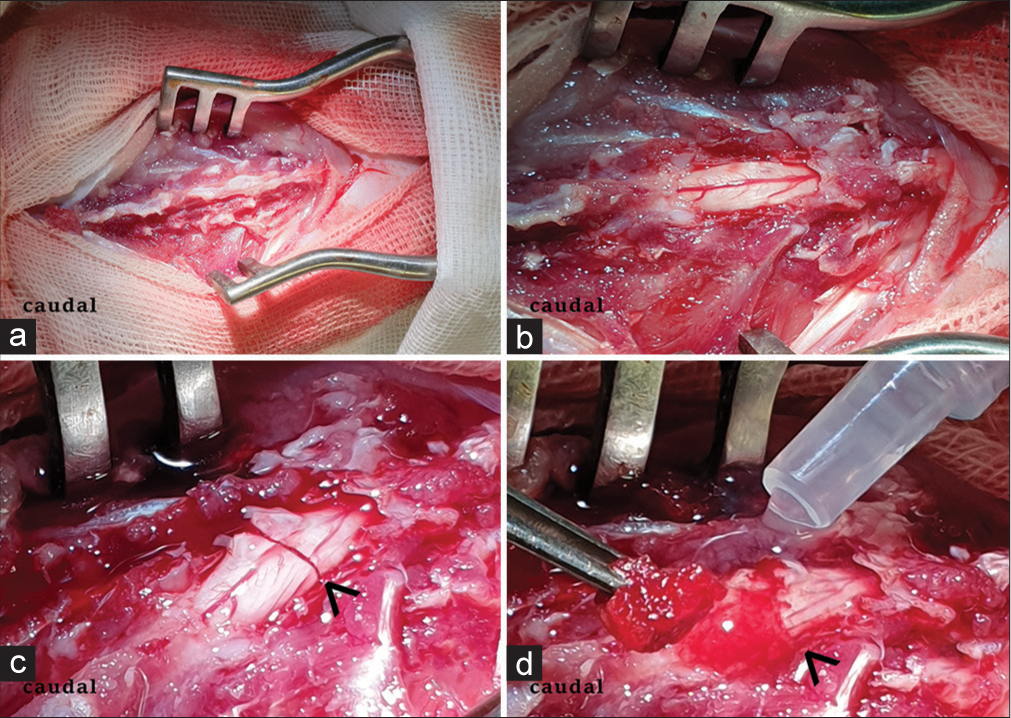
Healthy male rabbits (n = 20) with a mean weight of 2.5 kg were anesthetized by injection of Zoletil (Virbac, France) and Xylazine (Alfasan int., Netherlands) (1:4) after premedication with atropine sulfate and droperidol. A standard T9 laminectomy was performed. The dura mater was dissected longitudinally over a distance of 5–7 mm along the midline. We inserted a flexible spatula into the subdural space between the anterior surface of the spinal cord and the posterior surface of the vertebral bodies. After that, we transversely transected the spinal cord using scalpel No. 11 so that the scalpel rested on the spatula. Hemostasis was obtained by irrigation with a cold (8°C) isotonic saline solution. In the experimental group (n = 10), we injected PEG-chitosan at the site of injury [
Motor function was assessed on the modified Basso, Beattie, Bresnahan (mBBB) scale with a step test at a distance of 1 m and estimation of movements in joints. We tested the animals on a 1-m long horizontal ladder and a 2-cm ladder step. Sensory function was studied through needling the distal and proximal skin of the hind limbs, with quantification of reaction (0 = no reaction; 1 = vocalization (squeak, scream) in response to painful stimulation and/or attempt to remove the stimulus or escape from it (without limb movement); 2 = attempt to withdraw the limb; and 3 = active resistance to stimulus). We assessed diuresis, defecation, fullness of the bladder and intestine, and peristalsis.
Statistical analysis
The Kruskal–Wallis test was used for statistical evaluation. Calculations were made based on the clinical scores calculated on the mBBB scale for all postoperative days. Spearman’s correlation coefficient was used to assess the correlation of results between groups. Comparisons between the two groups at multiple time points were analyzed with a repeated measures analysis of variance. Differences between groups were considered significant at P < 0.05.
RESULTS
Immediately after surgery, we observed lower paraplegia with below-level anesthesia and pelvic dysfunction (retention) in all animals. Controls showed no improvement in motor and sensory function throughout the experiment [
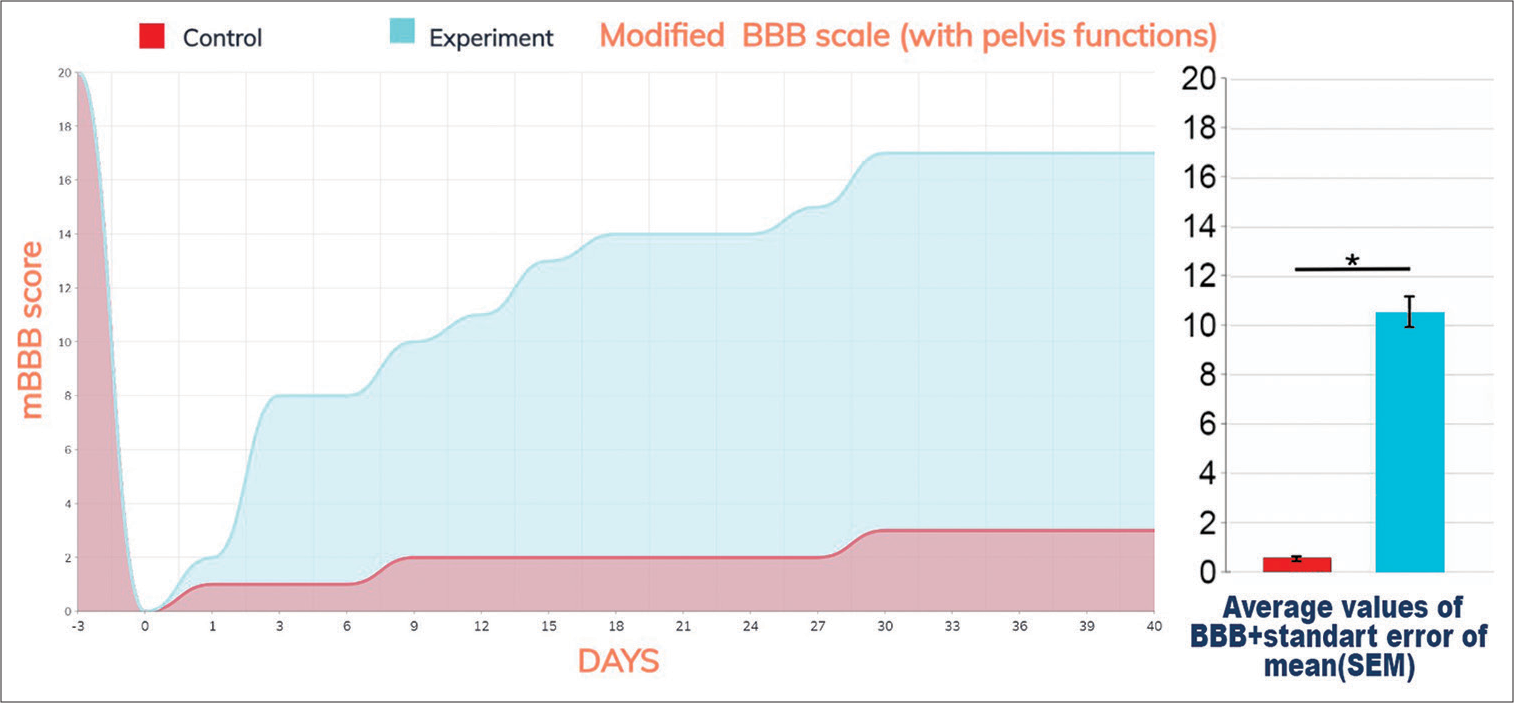
Figure 2:
Locomotion, sensory, and pelvis functions as scored on the modified modified Basso Beattie Bresnahan (mBBB) scale. Data in graphs are shown as means ± SEM. The values significantly differed from the control at P < 0.001 (P = 0.000251 for comparison of the experimental and control groups). In particular, notice the rapid onset of recovery on POD 2 versus none in controls.
Treated animals evinced the first signs of improvement after postoperative day (POD) 1. There were active movements of the hind legs in the horizontal plane (weak pulling of paws when moving) in one animal on POD 2. Five out of eight animals demonstrated strength in the hind limbs sufficient to push off and counter the experimenter’s hand between POD 7 and 10. Weak movements and recovery of pain sensitivity were observed in all animals by day 14. Animals could move with support on the hind legs but could not jump. Pelvic dysfunction was transient by this time, and spontaneous emptying of the bladder and large intestine occurred after a 1-day delay as a rule. Mild lower paraparesis persisted by the 21st day, but the range of movements increased significantly.
By the end of the experiment, slight paraparesis remained, but the range of movements allowed the animals to move freely, making small jumps [
Results were statistically highly significant (P = 0.000251, Spearman Coefficient: 0.91815).
Video 1
DISCUSSION
In this study, we confirm that fusogens are effective in rabbits, similar to all other species tested so far.[
In the current study, we did not tease apart the contribution of PEG and chitosan versus PEG versus chitosan alone, but this is clearly the objective of future studies. However, we notice that initial signs of recovery are seen after 24–48 h, in line with similar studies.[
CONCLUSION
In sum, our results add to the growing literature on the potential of fusogens to restore the anatomophysiologic continuity of a transected spinal cord and – as such – can be leveraged for several approaches to the treatment of spinal cord injury.
Ethical approval
The study protocol was approved by the Local Ethics Committee of the Stavropol State Medical University (№87, March 12, 2020). This trial was carried out in accordance with the ethical standards of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Canavero S, Ren X, editors. The technology of head transplantation. New York: NOVA Science; 2020. p.
2. Kim CY, Baek J, Canavero S, Ren X, editors. Spinal cord fusion in rodents and dogs. The technology of head transplantation. New York: NOVA Science; 2020. p. 83-92
3. Lebenstein-Gumovski MV, Botasheva VS, Kovalev DA, Shirov AM, Shatokhin AA, Shatokhin AV. Method for restoring the functions of the spinal cord after transection thereof, using PEG-chitosan conjugate, Russian Federation patent RU 2782119 C1, registration № 2021120198. 2021. p. (In Russ, Eng)
4. Lebenstein-Gumovski MV, Grin AA, Kovalev DA, Bashakhanov RM, Rasueva TS, Zharchenko AV. Method of treatment of spinal cord injury with restoration of its functions with PEG-chitosan conjugate «NEURO-PEG», Russian Federation patent RU 2801469 C1, registration No 2022128384. 2022. p. (In Russ, Eng)
5. Lebenstein-Gumovski MV, Bashakhanov RM, Kovalev DA, Zhirov AM, Shatohkin AA, Botasheva VS. Recovery of spinal cord functions after experimental complete crossection under the effect of chitosan polymeric compounds. Zh Vopr Neirokhir Im N N Burdenko. 2023. 87: 36-44 (In Russ)
6. Lebenstein-Gumovski MV, Botasheva VS, Kovalev DA, Shatokhin AA. Characterization of morphological and functional changes in the spinal cord after its section, under the influence of a hydrogel based on a modification of chitosan. Volgogr Nauchno Med Zh. 2021. 3: 37-42 (In Russ)
7. Ren X, Kim CY, Canavero S. Bridging the gap: Spinal cord fusion as a treatment of chronic spinal cord injury. Surg Neurol Int. 2019. 10: 51