- Department of Neurosurgery, Federal University of Ceará, Sobral, Ceará, Brazil
- Department of Surgical Oncology, Federal University of Ceará, Sobral, Ceará, Brazil
- Department of Surgical Oncology, Santa Casa de Misericórdia’s Hospital, Sobral, Ceará, Brazil
- Department of Neurosurgery, Santa Casa de Misericórdia’s Hospital, Sobral, Ceará, Brazil.
Correspondence Address:
José Elmano Silva, Department of Neurosurgery, Federal University of Ceará, Sobral, Ceará, Brazil.
DOI:10.25259/SNI_797_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: José Elmano Silva1, Gabriel de Almeida Monteiro1, Stefanie Torres e Silva1, Gabriel Marinheiro dos Santos Bezerra1, Joaquim Francisco Cavalcante-Neto1, Diego de Aragão Bezerra2,3, Janssen Loiola Melo Vasconcelos3, Paulo Roberto Lacerda Leal1,4. Chondrosarcoma secondary to hereditary multiple osteochondromas with spinal cord compression: A case report and systematic review. 03-Nov-2023;14:387
How to cite this URL: José Elmano Silva1, Gabriel de Almeida Monteiro1, Stefanie Torres e Silva1, Gabriel Marinheiro dos Santos Bezerra1, Joaquim Francisco Cavalcante-Neto1, Diego de Aragão Bezerra2,3, Janssen Loiola Melo Vasconcelos3, Paulo Roberto Lacerda Leal1,4. Chondrosarcoma secondary to hereditary multiple osteochondromas with spinal cord compression: A case report and systematic review. 03-Nov-2023;14:387. Available from: https://surgicalneurologyint.com/surgicalint-articles/12624/
Abstract
Background: Hereditary multiple osteochondromas (HMOs) are a rare genetic disorder characterized by the formation of multiple benign osteochondromas that can undergo malignant transformation into chondrosarcoma.
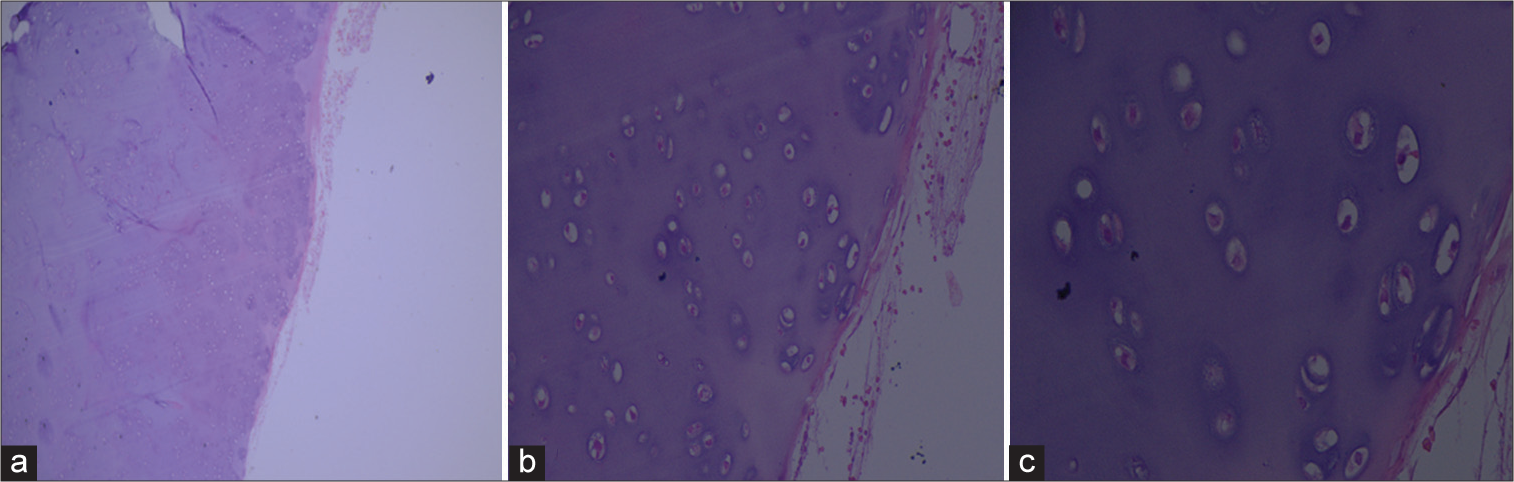
Case Description: A 24-year-old male with a history of HMO and osteochondroma surgery 4 years ago, presented with back pain and paresthesias. The magnetic resonance showed a right paravertebral infiltrating mass at the T12–L1 level causing spinal cord compression. Following en bloc resection of the tumor, the patient’s symptoms/ signs resolved. The final pathological diagnosis was consistent with a chondrosarcoma.
Conclusion: Chondrosarcomas secondary to HMO with spinal cord compression are rare. These patients often presenting with significant myelopathy/cord compression should undergo gross total resection where feasible to achieve the best outcomes.
Keywords: Diaphyseal aclasis, Hereditary multiple exostoses, Hereditary multiple osteochondromas, Secondary chondrosarcoma, Spinal cord compression
INTRODUCTION
Hereditary multiple osteochondromas (HMOs) are a rare autosomal dominant disease characterized by multiple osteochondromas.[
CASE PRESENTATION
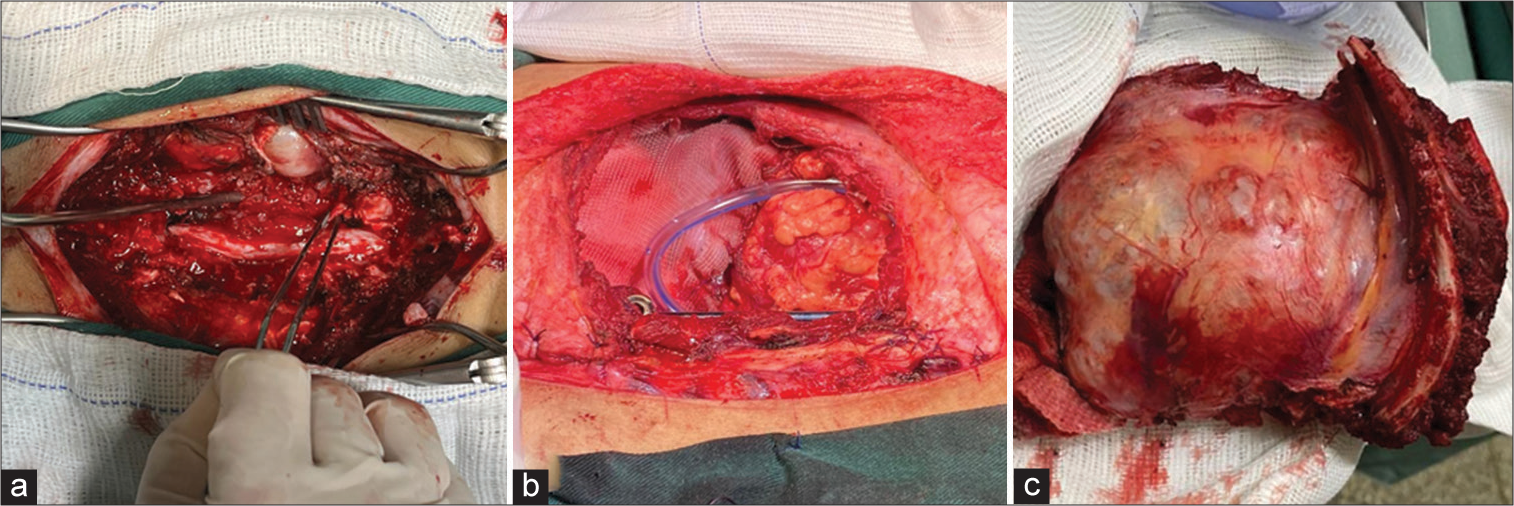
A 24-year-old male presented with back pain/paresthesias associated with a growing right-sided, posterior, infiltrating mass magnetic resonance (MR)-documented T12–L1 [
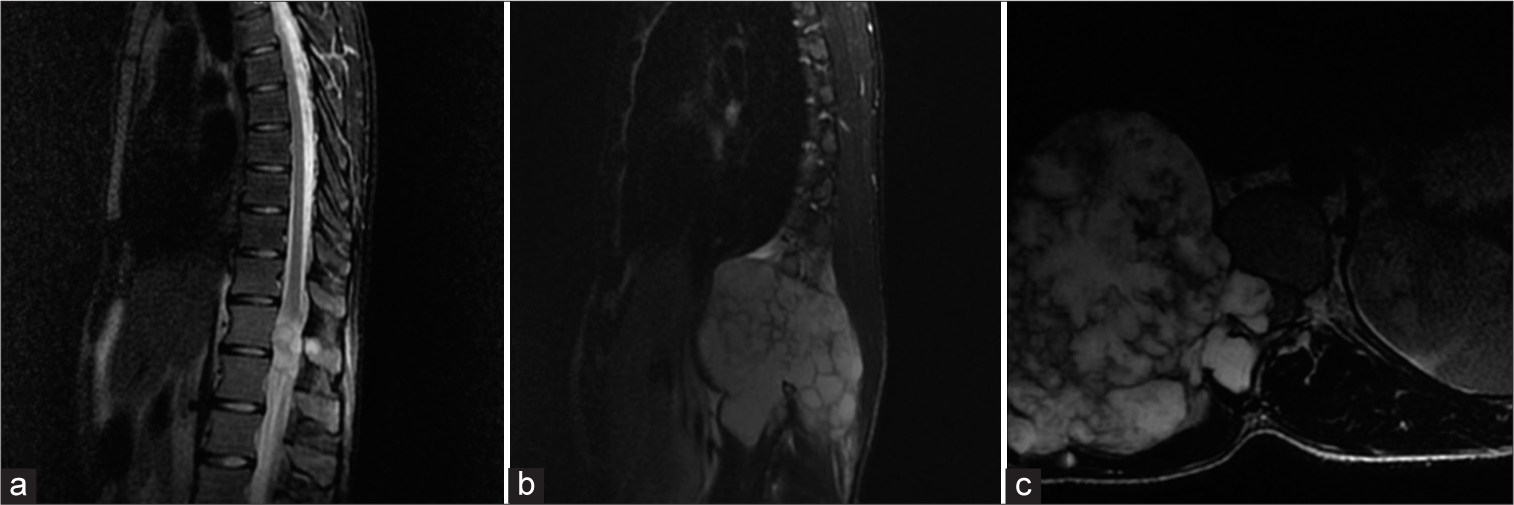
Figure 1:
Preoperative magnetic resonance imaging. (a) Midsagittal T2-weighted image (T2WI) with fat suppression revealing a hyperintense lesion causing medullary invasion at the T12–L1 levels. (b) Midsagittal T2WI with fat suppression showing a hyperintense expansive lesion in the right paravertebral area. (c) Axial T2WI with fat suppression revealed a significant spinal cord compression by the tumoral mass.
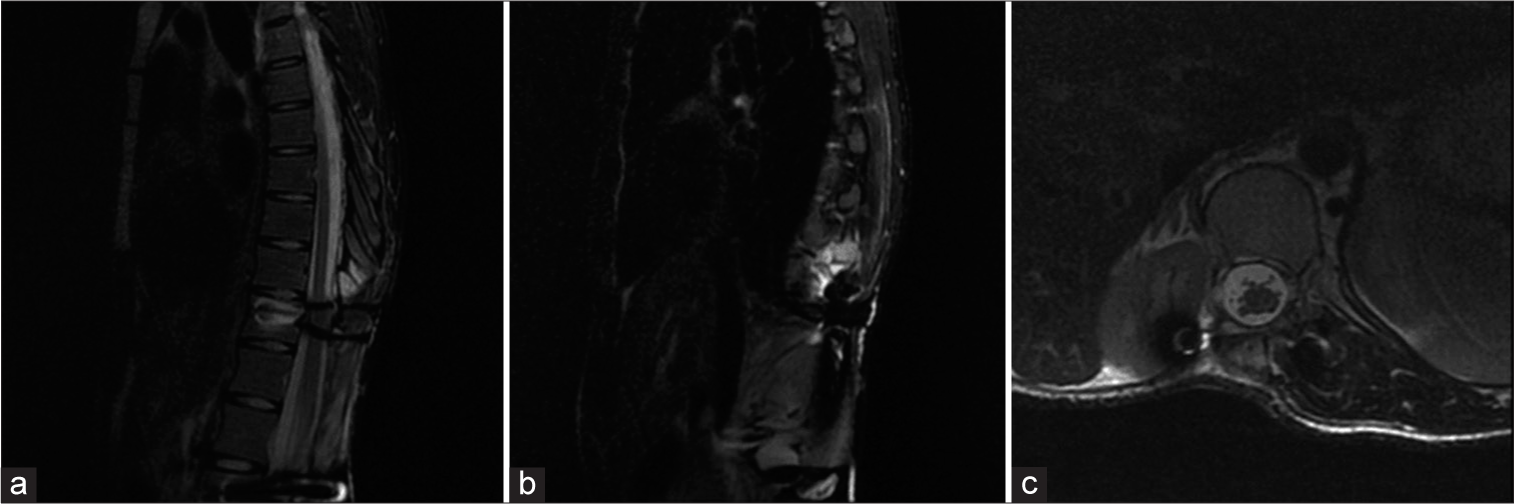
Figure 4:
Postoperative magnetic resonance imaging. (a and b) Midsagittal T2-weighted image (T2WI) with fat suppression showing complete resection of the tumoral components. (c) Axial T2WI with fat suppression revealing resolution of the spinal cord compression after the tumor removal, with the right kidney posterior displacement due to surgical procedure.
Literature epidemiology
HMO is a rare genetic disorder, with an incidence of 1.5% in the Western population.[
Complications of malignant transformation
Malignant transformation into secondary chondrosarcoma is still the most feared of all complications of HMO.[
Pathology of HMO
Secondary chondrosarcoma occurs in only 0.5–5% of cases and is characterized by malignant transformation of anterior cartilaginous lesions. Myxoid change may be characterized by the loss of the lacunar pattern with pleomorphic spindle or stellate cells floating in a myxoid stroma.[
Genetics of HMO
HMO is a genetically heterogeneous disease caused by tumor suppressor genes Exostosin-1 (EXT1) on chromosome 8, and Exostosin-2 (EXT2) on chromosome 11.[
Surgical resection to optimize survival of chondrosarcomas
Optimally, HMO lesions are treated surgically, and best with en bloc resection of thoracic tumors. Thoracic surgical options include; anterior, posterior, anterior approach first and posterior second, and finally, the posterior approach first followed by simultaneous anterior/posterior surgery.[
Resistance to radiation therapy (RT)
Chondrosarcomas are resistant to RT. Therefore, RT should only be reserved for those who are not candidates for surgery.[
CONCLUSION
Malignant transformation to chondrosarcoma may occur in patients with underlying HMO. Once, identified optimal outcomes are associated with gross total en bloc excision.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
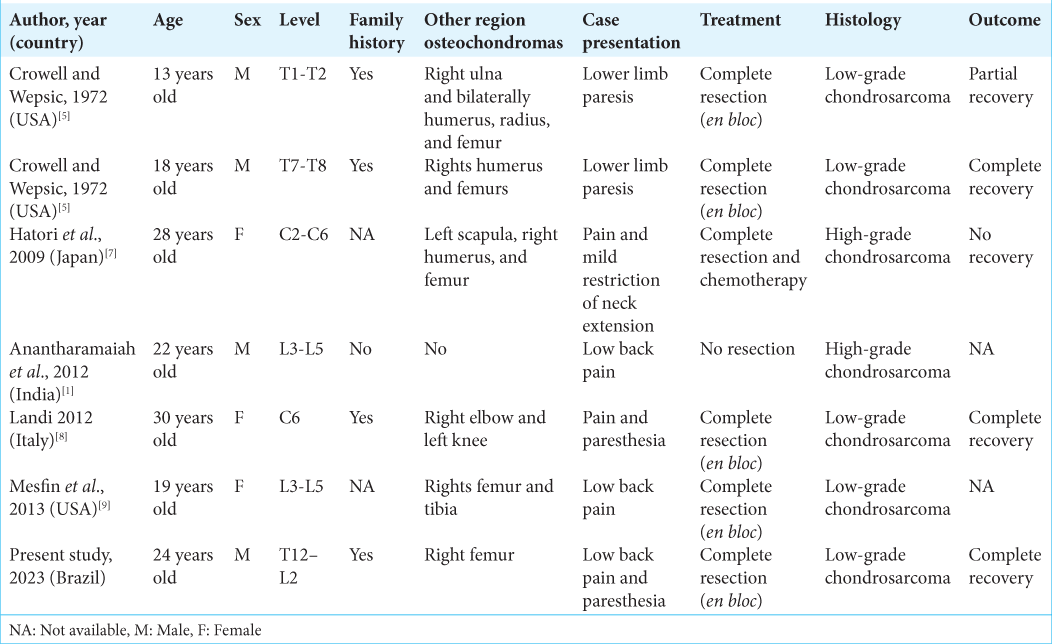
1. Anantharamaiah H, Kalyani R, Kumar ML, Manohar PV. Secondary chondrosarcoma of the lumbosacral region: Are any bones spared in the multiple hereditary exostoses?. J Clin Diagn Res. 2012. 6: 1778-80
2. Beltrami G, Ristori G, Scoccianti G, Tamburini A, Capanna R. Hereditary multiple exostoses: A review of clinical appearance and metabolic pattern. Clin Cases Miner Bone Metab. 2016. 13: 110-8
3. Boriani S, Bandiera S, Colangeli S, Ghermandi R, Gasbarrini A. En bloc resection of primary tumors of the thoracic spine: Indications, planning, morbidity. Neurol Res. 2014. 36: 566-76
4. Bovée JV. Multiple osteochondromas. Orphanet J Rare Dis. 2008. 3: 3
5. Crowell RM, Wepsic JG. Thoracic cord compression due to chondrosarcoma in two cousins with hereditary multiple exostoses. Report of two cases. J Neurosurg. 1972. 36: 86-9
6. Hameetman L, Bovée JV, Taminiau AH, Kroon HM, Hogendoorn PC. Multiple osteochondromas: Clinicopathological and genetic spectrum and suggestions for clinical management. Hered Cancer Clin Pract. 2004. 2: 161-73
7. Hatori M, Watanabe M, Tanaka Y, Ozawa K, Itoi E. Dedifferentiated chondrosarcoma of the cervical spine in a patient with multiple osteochondromas. Eur J Orthop Surg Traumatol. 2009. 19: 577-80
8. Landi A, Rocco P, Mancarella C, Tarantino R, Raco R. Malignant transformation of cervical ostochondroma in patient with hereditary multiple exostoses (HME): Case report and review of the literature. J Solid Tumors. 2012. 2: 63
9. Mesfin A, Ghermandi R, Castiello E, Donati DM, Boriani S. Secondary chondrosarcoma of the lumbar spine in hereditary multiple exostoses. Spine J. 2013. 13: 1158-9
10. Paulino Pereira NR, Janssen SJ, Stoop N, Hartveldt S, Chen YE, DeLaney TF. Physical function and quality of life after resection of mobile spine chondrosarcoma. Global Spine J. 2019. 9: 743-53
11. Pennington Z, Ehresman J, Pittman PD, Ahmed AK, Lubelski D, McCarthy EF. Chondrosarcoma of the spine: A narrative review. Spine J. 2021. 21: 2078-96