- Department of Neurosurgery, University of Erlangen-Nuremberg, Erlangen, Germany
- Department of Neurosurgery, Hannover Nordstadt Hospital, Hannover, Germany
- Department of Neuroradiology, University of Erlangen-Nuremberg, Erlangen, Germany
- Department of Neurosurgery, Bundeswehrkrankenhaus Ulm, Ulm, Germany
Correspondence Address:
Levent Tanrikulu
Department of Neurosurgery, University of Erlangen-Nuremberg, Erlangen, Germany
Department of Neurosurgery, Bundeswehrkrankenhaus Ulm, Ulm, Germany
DOI:10.4103/2152-7806.172534
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tanrikulu L, Hastreiter P, Arnd Dörfler, Buchfelder M, Naraghi R. Classification of neurovascular compression in glossopharyngeal neuralgia: Three-dimensional visualization of the glossopharyngeal nerve. Surg Neurol Int 24-Dec-2015;6:189
How to cite this URL: Tanrikulu L, Hastreiter P, Arnd Dörfler, Buchfelder M, Naraghi R. Classification of neurovascular compression in glossopharyngeal neuralgia: Three-dimensional visualization of the glossopharyngeal nerve. Surg Neurol Int 24-Dec-2015;6:189. Available from: http://surgicalneurologyint.com/surgicalint_articles/classification-of-neurovascular-compression-in-glossopharyngeal-neuralgia-three%e2%80%91dimensional-visualization-of-the-glossopharyngeal-nerve/
Abstract
Background:We introduce a method of noninvasive topographical analysis of the neurovascular relationships of the glossopharyngeal nerve (CN IX) by three-dimensional (3D) visualization. Patients with glossopharyngeal neuralgia (GN) resulting from neurovascular compression (NVC) were studied.
Methods:15 patients with GN were prospectively examined with 3D visualization using high-resolution magnetic resonance imaging with constructive interference in steady state (MR-CISS). The datasets were segmented and visualized with the real, individual neurovascular relationships by direct volume rendering. Segmentation and 3D visualization of the CN IX and corresponding blood vessels were performed. The 3D visualizations were interactively compared with the intraoperative setup during microvascular decompression (MVD) in order to verify the results by the observed surgical-anatomical findings.
Results:15 patients (female/male: 5/10) were examined. All of them underwent MVD (100%). Microvascular details were documented. The posterior inferior cerebellar artery (PICA) was the most common causative vessel in 12 of 15 patients (80%), the vertebral artery (VA) alone in one case (6.7%), and the combination of compression by the VA and PICA in 3 patients (13.3%). We identified three distinct types of NVC within the root entry zone of CN IX.
Conclusion:3D visualization by direct volume rendering of MR-CISS data offers the opportunity of noninvasive exploration and anatomical categorization of the CN IX. It proves to be advantageous in supporting to establish the diagnosis and microneurosurgical interventions by representing original, individual patient data in a 3D fashion. It provides an excellent global individual view over the entire neurovascular relationships of the brainstem and corresponding nerves in each case.
Keywords: Classification, glossopharyngeal nerve, glossopharyngeal neuralgia, image processing, neurovascular compression
INTRODUCTION
The glossopharyngeal nerve (CN IX) at the ventrolateral medulla oblongata features a complex formation in the posterior cranial fossa. The CN IX emerges from the retro-olivary sulcus in the region of the flower basket of Bochdalek and superiorly courses to the vagus nerve to the jugular foramen. At the root entry zone (REZ) of CN IX blood vessels such as the posterior inferior cerebellar artery (PICA) and the vertebral artery (VA) may compress the CN IX resulting in neurovascular compression (NVC).[
CLINICAL MATERIAL AND METHODS
Clinical data
We studied 15 patients with GN [
Imaging
Imaging was performed with a Siemens MR Magnetom Sonata 1.5 Tesla scanner. MRI with constructive interference in steady state (MR-CISS) as highly T2-weighted sequence was used (Time of repetition/Time of echo (TR/TE) 12.2/5.9 ms, flip angle 70°, acquisition time 5 min, matrix 512 × 512, and slice thickness 0.4 mm).[
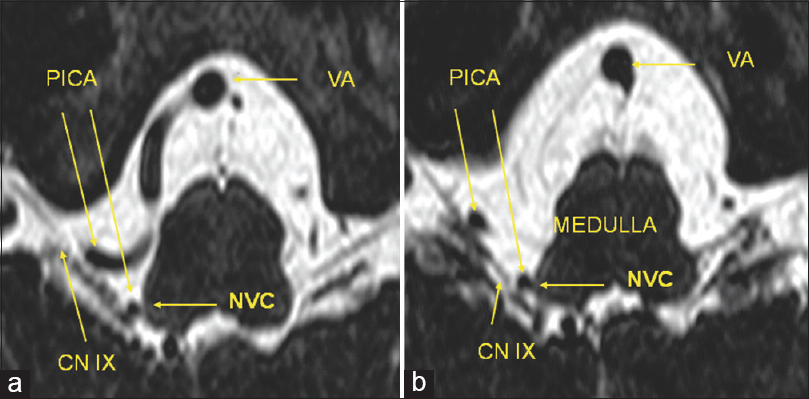
Figure 1
High-resolution magnetic resonance imaging with constructive interference in steady state sequence with neurovascular compression of the right-sided glossopharyngeal nerve. (a and b) A right-sided posterior inferior cerebellar artery-loop originating from the vertebral artery and coursing into the root entry zone of the glossopharyngeal nerve at the ventrolateral medulla oblongata. VA: Vertebral artery, PICA: Posterior inferior cerebellar artery, CN IX: Glossopharyngeal nerve, NVC: Neurov1ascular compression
Image processing
Image processing consists of segmentation and direct volume rendering. Segmentation consists of the coarse extraction of relevant structures [
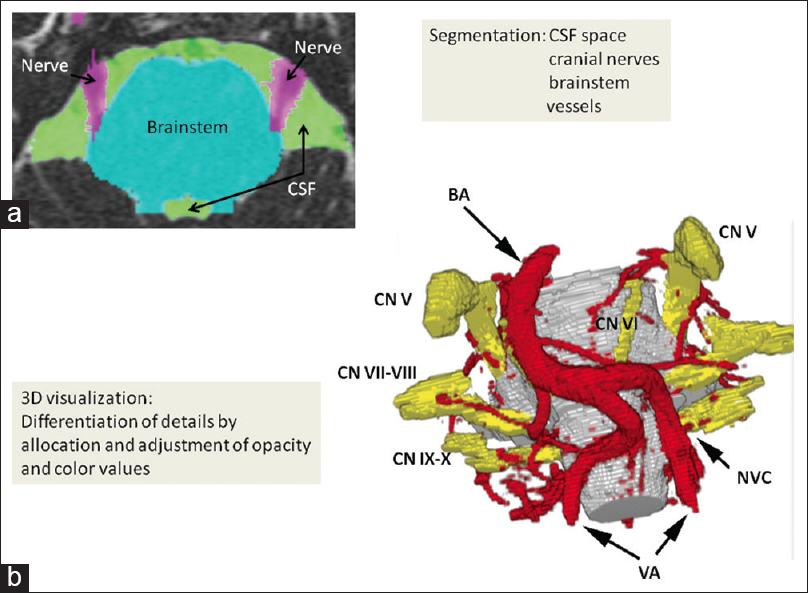
Figure 2
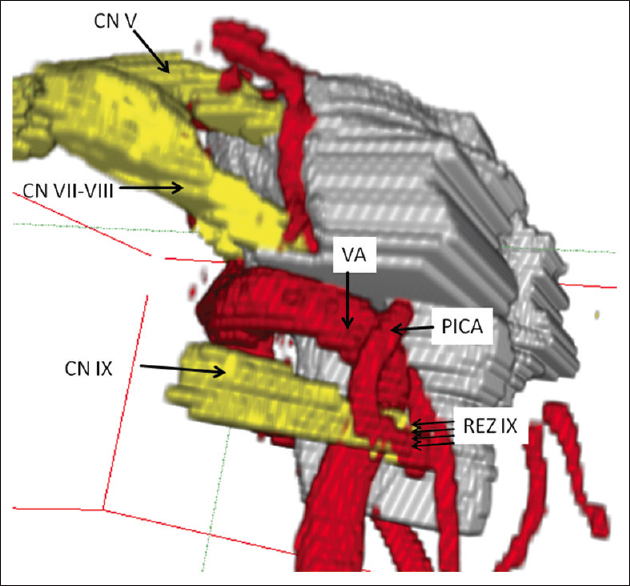
Workflow of image processing. (a) Segmentation of magnetic resonance imaging with constructive interference in steady state sequence: Each anatomical subvolume, cerebrospinal fluid space including vessels (green color), cranial nerves (purple color), and the brainstem (turquoise color) are segmented. (b) After segmentation of each subvolume, the acquired volumes are adjusted and allocated to opacity and color values, so that a real, individual three-dimensional representation based on the anatomical magnetic resonance imaging with constructive interference in steady state data is visualized. This three-dimensional visualization object can be rotated in each direction to observe the interesting topographical regions in a noninvasive way (BA: Basilar artery, VA: Vertebral artery, CN V: Trigeminal nerve, CN VI: Abducens nerve, CN VII-VIII: Facial-vestibulocochlear nerve, CN IX-X: Glossopharyngeal-vagus nerve)
RESULTS
The MR-CISS images clearly depicted the CN IXs and corresponding vessels within the hyperintense CSF volume. We could distinguish nerves from vessels on the basis of signal intensity, anatomical characteristics, and the anatomical course in the MR-CISS. The interactive 3D visualization demonstrated the spatial relationships between the CN IX and the corresponding vessels in all patients (100%); [Figures
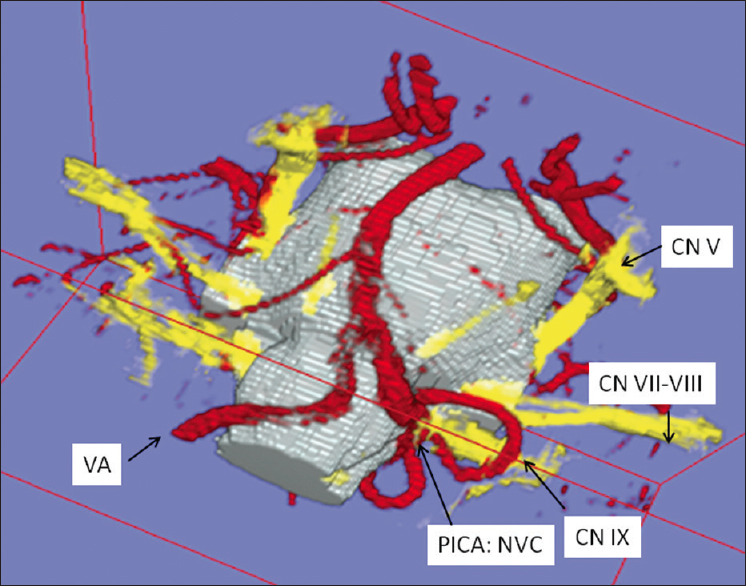
Figure 3
Three-dimensional visualization in left-sided glossopharyngeal neuralgia. A posterior inferior cerebellar artery-loop originating from the left vertebral artery courses over the left glossopharyngeal nerve and performs neurovascular compression at the glossopharyngeal root entry zone at the ventrolateral medulla oblongata (VA: Vertebral artery, PICA: Posterior inferior cerebellar artery, CN IX: Glossopharyngeal nerve)
Figure 4
Lateral view of three-dimensional visualization in left-sided glossopharyngeal neuralgia. neurovascular compression is performed by a “sandwich” compression of the root entry zone of the glossopharyngeal nerve by the left-sided vertebral artery and a branching loop of posterior inferior cerebellar artery (CN IX: Glossopharyngeal nerve, REZ: Root entry zone, CN VII-VIII: Facial-vestibulocochlear nerve, CN V: Trigeminal nerve, VA: Vertebral artery, PICA: Posterior inferior cerebellar artery)
Figure 5
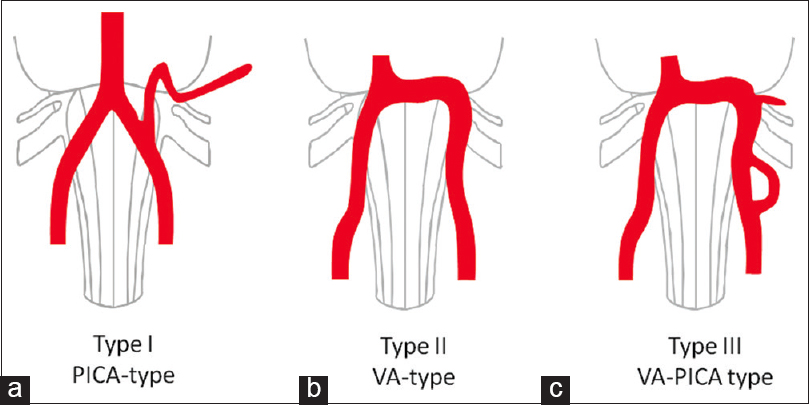
Types of neurovascular compression of the glossopharyngeal nerve. Type I as the most frequent one is caused by the posterior inferior cerebellar artery, which travels first cranially and performs neurovascular compression by a downward directed convex dome more distally. Type II is performed by the “shoulder” of the vertebral artery. Type III is performed by the combination of compression by the vertebral artery and the branching posterior inferior cerebellar artery
Figure 6
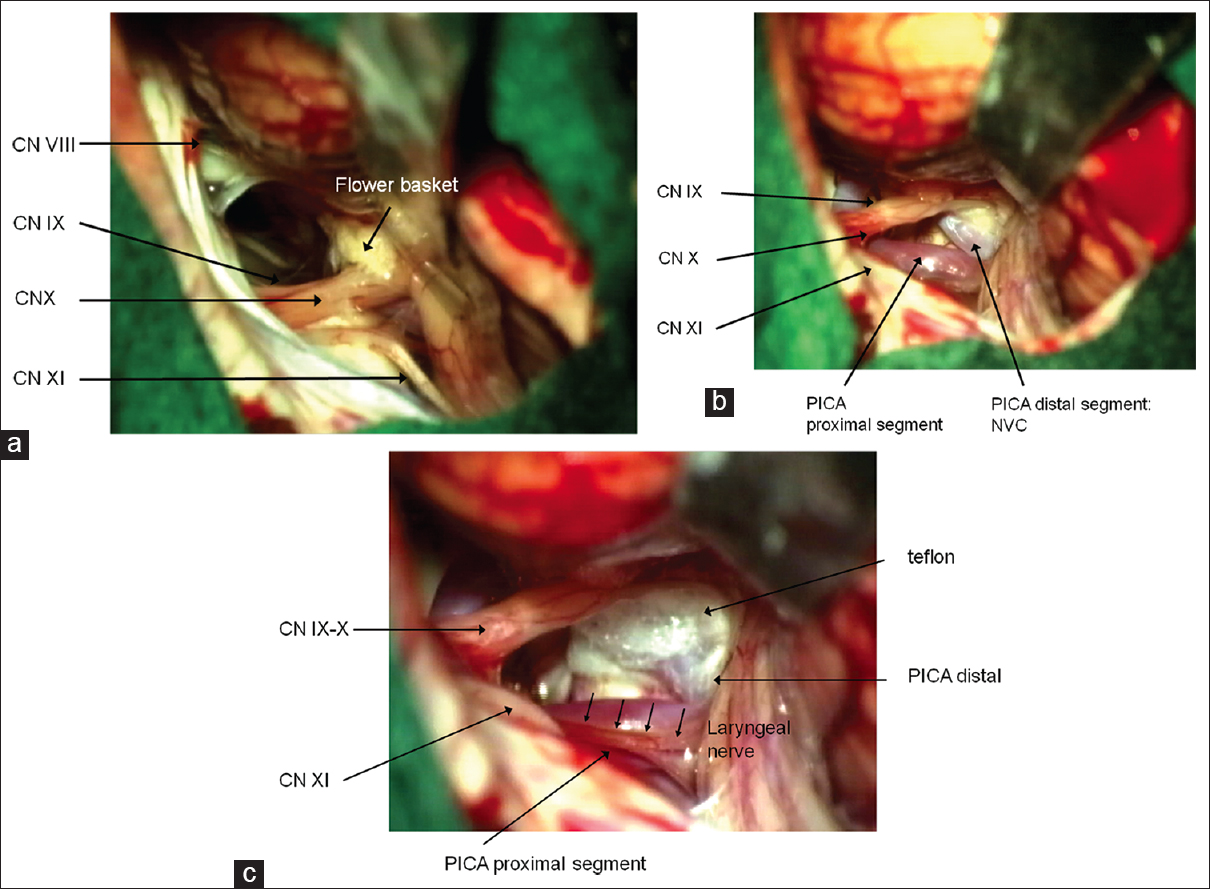
Surgical aspect of microvascular decompression by the interpolation of teflon between the nerve and the causative vessel. The surgical microphotographs correspond to three-dimensional visualization in
Five patients showed right-sided neuralgia (33%) and 10 patients showed left-sided neuralgia (67%). In this series, 14 of 15 patients (93.3%) become pain-free in the postoperative period (follow-up 6–48 months), whereas 1 patient (6.7%) showed pain-reduction instead of pain-freedom. One patient showed transient dysphagia (6.7%), and another one showed transient CSF leak (6.7%;
DISCUSSION
2D representations do not give sufficient information over the spatial relationships of nerves and vessels. Therefore, we introduced and applied the technique of direct volume rendering to obtain 3D visualization of the CN IX. For optimized imaging of the CN IX, the MR-CISS sequence was applied. This imaging sequence improved the contrast between neighboring structures mostly allowing differentiation of nerves and vessels within the CSF space. Isotropic voxel sizes of the volume data were used to improve the interplane resolution. This ensured equal high-resolution in the axial, coronal, and sagittal slices. The lateral medulla oblongata containing the CN IXs is demonstrated very well in contrast to the cisternal compartiments around the midline. In the axial slice images of MR-CISS, we were able to differentiate the CN IXs, as well as all other remaining cranial nerves V-XI. In each case, we were able to visualize the CN IXs. Interactive 3D visualization is a prerequisite for comprehensive analysis and understanding of volumetric data from MRI. The segmentation of MR-CISS takes about 1–2 h, and the adjustment of transfer functions referring to direct volume rendering takes <3 min. The visualization of the neurovascular structures of the CN IX was useful in preoperative planning and during surgery. We applied the 3D visualization intraoperatively and analyzed microsurgical details during MVD. 3D visualization of neurovascular structures provides a great deal of topographic information before surgery. A classification of the vascular courses causing NVC in GN does not exist until now. This classification is considered to be necessary for a reproducible and standardized evaluation in establishing the diagnosis, planning, and performing surgery. The suggested classification derived from original individual data was able to cover all anatomical situations, which we found in this analysis. We did not find any tumorous mass within the surface of the lateral medulla oblongata neither in the preoperative, diagnostic MRI nor during surgery, which could also be another possible cause of GN.[
We can confirm that MVD is the treatment of choice for medication-refractory GN.[
CONCLUSIONS
We presented a method of noninvasive evaluation of the relationships of the CN IX and the corresponding blood vessels with high-resolution MRI using MR-CISS and 3D visualization based on direct volume rendering in patients with GN. Distinct types of courses of vessels causing NVC are described. Several characteristics for the identification of NVC were described allowing a reproducible and standardized evaluation of the neurovascular relationships. We suggest this method of noninvasive anatomical exploration of the CN IX as very useful for topographic classification of the variable vascular compression courses along the REZ of the CN IX in order to support diagnostic and surgical procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Hastreiter P, Naraghi R, Tomandl B, Bonk A, Fahlbusch R. Analysis and 3-dimensional visualization of neurovascular compression syndromes. Acad Radiol. 2003. 10: 1369-79
2. . Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2 nd edition. Cephalalgia. 2004. 24: 9-160
3. Jannetta PJ, Segal R, Wolfson SK, Dujovny M, Semba A, Cook EE. Neurogenic hypertension: Etiology and surgical treatment. II. Observations in an experimental nonhuman primate model. Ann Surg. 1985. 202: 253-61
4. Kalkanis SN, Eskandar EN, Carter BS, Barker FG. Microvascular decompression surgery in the United States, 1996 to 2000: Mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery. 2003. 52: 1251-61
5. Leal PR, Hermier M, Froment JC, Souza MA, Cristino-Filho G, Sindou M. Preoperative demonstration of the neurovascular compression characteristics with special emphasis on the degree of compression, using high-resolution magnetic resonance imaging: A prospective study, with comparison to surgical findings, in 100 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Acta Neurochir (Wien). 2010. 152: 817-25
6. Levy EI, Scarrow AM, Jannetta PJ. Microvascular decompression in the treatment of hypertension: Review and update. Surg Neurol. 2001. 55: 2-10
7. Marion DW, Jannetta PJ. Use of perioperative steroids with microvascular decompression operations. Neurosurgery. 1988. 22: 353-7
8. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: Lessons learned after 4400 operations. J Neurosurg. 1999. 90: 1-8
9. Naraghi R, Hastreiter P, Tomandl B, Bonk A, Huk W, Fahlbusch R. Three-dimensional visualization of neurovascular relationships in the posterior fossa: Technique and clinical application. J Neurosurg. 2004. 100: 1025-35
10. Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995. 36: 64-8
11. Rozen TD. Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin. 2004. 22: 185-206
12. Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia: A study of 217 cases. Arch Neurol. 1981. 38: 201-5
13. Sampson JH, Grossi PM, Asaoka K, Fukushima T. Microvascular decompression for glossopharyngeal neuralgia: Long-term effectiveness and complication avoidance. Neurosurgery. 2004. 54: 884-9