- Department of Neurosurgery, Federal Center of Neurosurgery, Tyumen, Russian Federation,
- Department of Neurosurgery, Hospital General Universitario de Alicante, Alicante, Spain,
- Department of Neurosurgery, First Moscow Medical University, Moscow, Russian Federation,
- Department of Neurosurgery, Hospital General Universitario de Elche, Elche, Spain,
- Department of Neurosurgery, San Filippo Neri Hospital/ASLRoma1, Roma, Italy,
- Department of Neurosurgery, Mackenzie Evangelical University Hospital, Curitiba, Parana, Brazil,
- Department of Neurosurgery, Institute of Security and Social Services for State Workers (ISSSTE), Mexico City, Mexico,
- Laboratory of Microsurgical Neuroanatomy, Second Chair of Gross Anatomy, School of Medicine, University of Buenos Aires, Buenos Aires, Argentina
- Department of Neurosurgery, Hospital Padilla de Tucuman, Tucuman, Argentina
- 0Department of Neurosurgery, San Fernando Hospital, San Fernando, Argentina.
Correspondence Address:
Matias Baldoncini, Department of Neurosurgery, San Fernando Hospital, San Fernando, Argentina.
DOI:10.25259/SNI_210_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Albert Sufianov1, Pablo Gonzalez-Lopez2, Keith Simfukwe3, Carlos Martorell-Llobregat4, Iurii A. Iakimov3, Rinat A. Sufianov3, Luciano Mastronardi5, Luis A. B. Borba6, Carlos Castillo Rangel7, Valeria Forlizzi8, Alvaro Campero9, Matias Baldoncini10. Clinical and anatomical analysis of the epileptogenic spread patterns in focal cortical dysplasia patients. 18-Aug-2023;14:291
How to cite this URL: Albert Sufianov1, Pablo Gonzalez-Lopez2, Keith Simfukwe3, Carlos Martorell-Llobregat4, Iurii A. Iakimov3, Rinat A. Sufianov3, Luciano Mastronardi5, Luis A. B. Borba6, Carlos Castillo Rangel7, Valeria Forlizzi8, Alvaro Campero9, Matias Baldoncini10. Clinical and anatomical analysis of the epileptogenic spread patterns in focal cortical dysplasia patients. 18-Aug-2023;14:291. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12507
Abstract
Background: Focal cortical dysplasia (FCD) is one of the main causes of intractable epilepsy, which is amendable by surgery. During the surgical management of FCD, the understanding of its epileptogenic foci, interconnections, and spreading pathways is crucial for attaining a good postoperative seizure free outcome.
Methods: We retrospectively evaluated 54 FCD patients operated in Federal Center of Neurosurgery, Tyumen, Russia. The electroencephalogram findings were correlated to the involved brain anatomical areas. Subsequently, we analyzed the main white matter tracts implicated during the epileptogenic spreading in some representative cases. We prepared 10 human hemispheres using Klinger’s method and dissected them through the fiber dissection technique.
Results: The clinical results were displayed and the main white matter tracts implicated in the seizure spread were described in 10 patients. Respective FCD foci, interconnections, and ectopic epileptogenic areas in each patient were discussed.
Conclusion: A strong understanding of the main implicated tracts in epileptogenic spread in FCD patient remains cardinal for neurosurgeons dealing with epilepsy. To achieve meaningful seizure freedom, despite the focal lesion resection, the interconnections and tracts should be understood and somehow disconnected to stop the spreading.
Keywords: Epilepsy, Focal dysplasia, Seizure spreading, White matter tract
INTRODUCTION
Focal cortical dysplasia (FCD) is one of the leading causes of medically intractable epilepsy during the first and second decades of life.[
Despite the modern magnetic resonance imaging (MRI) modalities, FCD lesion boundaries, particularly in FCD Type I and Type IIa, are often complex to delineate. It is, however, incumbent that neurosurgeons not only acquire a firm understanding of the focal lesions, but also of their potential epileptogenic spreading.
The existence of enhanced connectivity within the region of the lesion and ectopic EZs means a great challenge in the surgical management of patients with intractable epilepsy. Due to the EZ location within and beyond the perilesional boundaries, patients may show symptoms nonrelated to the primary dysplastic area, as interconnected epileptic networks play a cardinal role in the onset and propagation of ictal activity.[
The process to accurately diagnose and localize the epileptic foci, with their epileptic zones, has greatly improved over the years. The cumulative use of invasive and noninvasive imaging techniques, patient’s clinical presentation, and electroencephalogram (EEG) studies represents a cardinal component in the surgical management of FCD. Contemporary epilepsy surgery has shifted the paradigm into anatomical pathways of seizure spread. Therefore, it is not surprising the large number of reports highlighting the correlation between EEG findings and the involved anatomical areas, driving physicians dealing with epilepsy patients to gain an in-depth understanding of the implicated ictal and inter-ictal EZs.[
Thus, the understanding of the seizure spreading pathophysiology from the FCD areas through its EZ and even to more remote cortical areas[
MATERIALS AND METHODS
Patients
We retrospectively analyzed the clinical records, as well as the pre and postoperative MRI images of a series of 54 FCD patients admitted for surgery due to intractable epilepsy at the Federal Center of Neurosurgery Tyumen, Russia, from September 2020 to July 2021. Ten out of those 54 patients were selected as the most representative cases to describe their clinical, electrophysiological, surgical, and anatomical features.
EEG evaluation
Patients were evaluated by an experienced neurophysiologist using long-term video EEG monitoring, through either a Nicolet One 32-channel device (stationary) (USA), bedside EEG system Nicolet ONE 16-channel and 32-channel (USA) device, a BE Plus (128-channel) EBNeuro/Ates (Italy) device, a Cadwell Easy III 64-channel device (USA), or invasive video-electrocorticography-monitoring. Ictal and inter-ictal recordings were then saved, evaluated, and interpreted. The aim of the detailed evaluation, together with other tests, was to identify the kind of seizures (generalized vs. focal vs. multifocal) and to determine the seizure onset zone (SOZ). The following key areas were also sought for: (1) SOZ, (2) irritative zone, (3) symptomatogenic zone (area that produces ictal symptoms), (4) functional deficit zone – interictal (functionally abnormal area), and (5) eloquent cortex – area of highly functional cortex.
Presurgical evaluation
All patients were symptomatic for FCD with confirmed resistance to anti-epileptic drugs (AED). A strict presurgical protocol included a specialized evaluation by neuroepileptologists, neuropsychiatrists, pediatricians, and neuroradiologists. Informed consent for surgery was obtained. A preoperative 1.5 Tesla MRI was routinely acquired in all FCD patients. In case, a more precise imaging evaluation was required, 3.0 T MRI studies were performed. The following MRI features were particularly sought: local thickening/ thinning or atrophy of cortex, blurred borders between gray and white matter, presence of irregularly shaped gyri, heterotopy (shifting of gray matter towards the ventricles), transmantle dysplasia (the spread of gray matter activity to the brain ventricles through white matter fibers), hypo-intensity of the signal from white matter on T1-weighted imaging, hyper intensity of the signal from white matter on T2 and fluid attenuated inversion recover modes, complex “expansion of the subarachnoid space, and spreading deeper into the cortex.” MRI baring the listed features was regarded as “MRI Positive,” while MRI was considered “Negative” when images showed no obvious lesions.
Surgery
All procedures were carried out by the first author. A frameless navigation system was used to delineate the optimal craniotomy in each case. Microsurgical removal of epileptogenic tissue was performed; under the guidance of intraoperative ultrasound (iUS). The FCD areas were identified as hyper-echoic relative to the healthy brain tissue, with uneven, fuzzy, and irregularly-shaped contours, by iUS. The use of iUS had the following main objectives: (1) localize dysmorphic brain tissue before and after opening the dura mater, due to shifting of anatomical structures once the dura is opened, (2) localize the pathological focus, (3) determine the structure and echogenicity of dysplastic tissue in relation to the surrounding normal brain, (4) delineate the contours of the abnormal/pathological tissue; (5) measure the dimensions of dysplasia; and (6) evaluate the effectiveness of the iUS to accurately delineate the area of resection, to optimize postoperative outcome. The FCD located within eloquent areas and involving white matter tracts within the EZ was safely resected and disconnected using multiple subpial resection techniques, with the aim of transecting the associative connections, trying to spare the projection fibers and vessels intact, and therefore increasing the chances of preserving the cortical function. Cortical mapping was carried out when the FCD was located close to highly functional areas, based on anatomy and clinical symptoms. Patients were followed up at 1st, 3rd, 6th months, and 1 year after surgery.
Correlation between EEG and anatomical areas
A correlation between the ictal and interictal EEG registered patterns and the involved anatomical areas was performed for each of the 54 cases included in the study, and based on the previous published data[
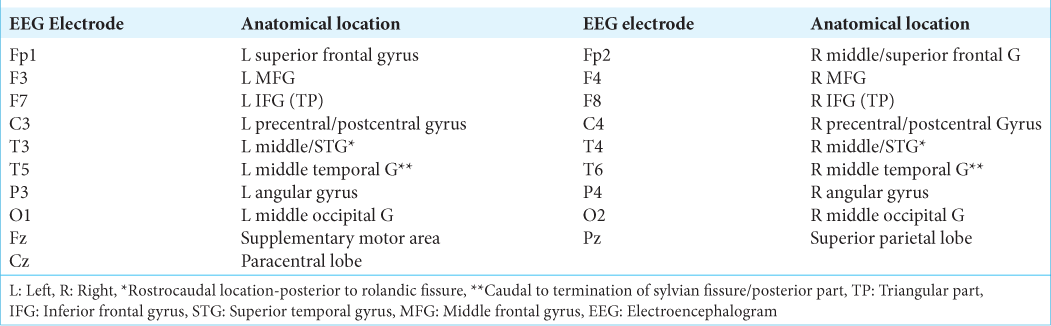
Figure 1:
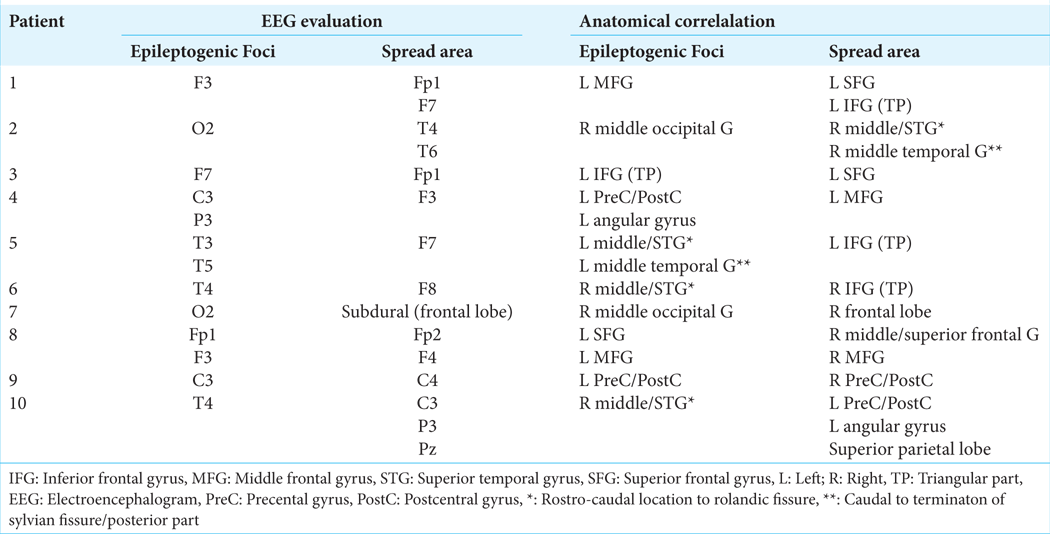
Correlation between electroencephalogram electrodes and cortical anatomy. Superior and lateral view of the brain surface (left side). Fp1: L Superior frontal gyrus, F3: L Middle frontal gyrus, F7: L Inferior frontal gyrus (TP), C3: L Precentral/Postcentral gyrus, T3: L Middle/Superior temporal gyrus*, T5: L Middle temporal gyrus, P3: L Angular gyrus, O1: L Middle occipital gyrus, Fz: Supplementary motor area, Cz: Paracentral lobe, Fp2: R Middle/ Superior frontal G, F4: R Middle Frontal gyrus, F8: R Inferior Frontal gyrus (TP), C4: R Precentral/Postcentral gyrus, T4: R Middle/superior temporal gyrus*, T6: R Middle temporal G **, P4: R Angular gyrus, O2: R Middle occipital gyrus, Pz: Superior parietal gyrus, L: Left, R: Right, *: Rostro-caudal location-posterior to rolandic fissure; **: Caudal to termination of sylvian fissure/posterior part. TP: Triangular part
Diffusion tensor imaging (DTI) methodology and description
A male right-handed patient was evaluated using a 1.5 T MRI epilepsy protocol. DTI-MRI in axial planes was also included in the study. The tractography of each tract was reconstructed using the iPlanet/BrainLab software. Afterward, the 3D reconstruction of the brain and each of the tracts were displayed, using the BrainLab workstation to illustrate the most common tracts involved on the epileptogenic activity spreading in our series.
Cadaver preparation and dissection
After seeking institutional approval, 10 cadaveric hemispheres were harvested during autopsy for teaching purposes. All 10 hemispheres were obtained after immersion in a 10–15% formalin solution for a minimum period of 4 weeks. After arachnoid and vessels removal, the brains were frozen during 2 weeks (−18°C). The selected cortical regions were marked and the white fiber tracts were dissected using the fiber dissection technique, aided by an exoscope (Karl Storz 4K 3D VITOM exoscope) and microscope (Carl Zeiss OPMI Vario S8 Microscope) [
RESULTS
Patients’ evaluation and surgery
Of the 10 patients included, six patients were female (60%) and four were male (40%). The mean age was 10.42 years (Standard Deviation ± 7.63). All patients were symptomatic for FCD, taking 2 or more AED without seizure relief. MRI was positive for FCD in all cases studied. EEG ictal and interictal evaluation (scalp and invasive) was performed in each case, confirming the origin and spread of the epileptiform activity in each of them. All patients underwent surgery, performing lesionectomy plus corticectomy with disconnection of fibers connecting to white matter tracts in seven cases, temporal lobectomy in two patients, and callosal disconnection in one case. The basal features, symptoms, AED, MRI results, EEG recording, type of surgery, and functional outcome (employing Engel scale) are summarized in
Anatomical–electrophysiological correlation
A correlation between the ictal and interictal EEG registered patterns and the involved anatomical areas has been performed for each patient [
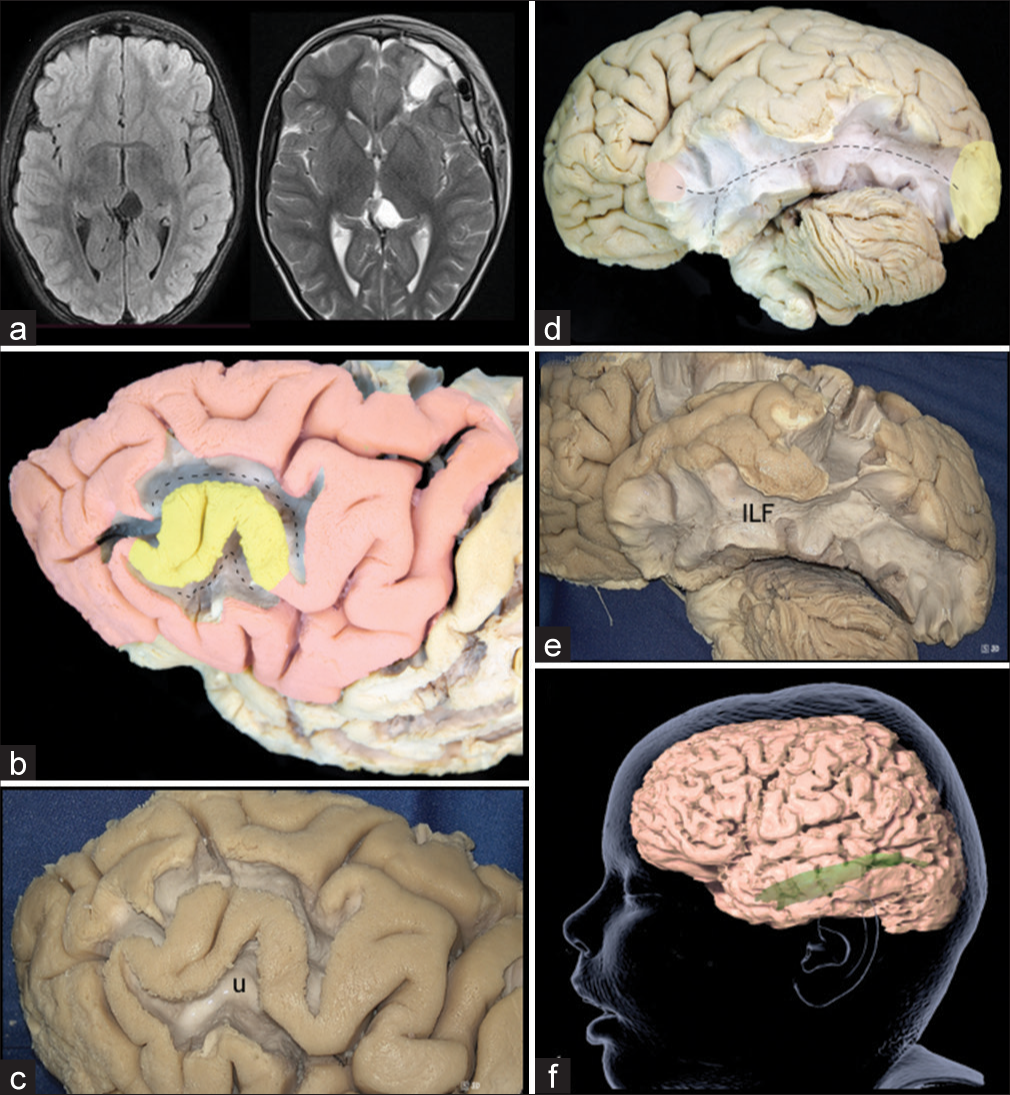
Case 1: “U” Fibers spread
In Case 1, the EEG showed an epileptogenic activity focus in the middle frontal gyrus (MFG), spreading to the superior and inferior frontal gyri, suggesting U-fibers involvement, as these are connecting adjacent giry. The short association U-fibers connect adjacent gyri running just below the deepest parts of the sulci. It is through these connections networks that FCD EZ spreads to adjacent brain areas. They arise from a gyrus, curve 180° into the sulcus depth, and then subsequently terminate into the adjacent gyrus neurons. In the case of the MFG, the U-fibers connect it with the superior frontal, inferior frontal and precentral gyrus, and also with other intra-gyral MFG areas[
Figure 3:
(a) Preoperative and postoperative maganetic resonance imaging (MRI) in a patient with focal cortical dysplasia (FCD) located in the left middle frontal gyrus (MFG). (b) Lateral view of the left frontal lobe, after dissecting of the U-fibers around the MFG. The epileptogenic zones (EZ) spread from the MFG to the inferior frontal gyrus and superior frontal gyrus through the U-fibers. Yellow: primary FCD EZ, Red: ectopic EZ, Discontinuous line: U-fibers. (c) Lateral view of the left frontal lobe, after dissecting the U-fibers around the MFG. u: U-fibers. (d) Lateral view of the left hemisphere, after removing the gray matter and U-fibers, exposing the ILF, which connects the EZ located in the occipital pole with the superior and medial temporal gyrus. Yellow: primary FCD EZ, Red: ectopic EZ. Discontinuous line: ILF. (e) Lateral view of the left hemisphere, after dissecting the ILF. ILF: Inferior Longitudinal Fascicle. (f) Tractography of the ILF.
Case 2: Inferior longitudinal fascicle (ILF) fibers spread
Case 2 EEG recorded epileptic foci in the right occipital pole (O2) and EZ probably spreading through the ILF to the right temporal lobe (T4, T6). The ILF is a long associative white matter tract that connects the anterior part of the temporal lobe to the occipital lobe and is composed by two components: a direct pathway and an indirect one. The indirect pathway is formed by U-shaped fibers that connect the adjacent gyri of the lateral occipitotemporal cortices to form the occipital-temporal projection system, described by Herbet et al.[
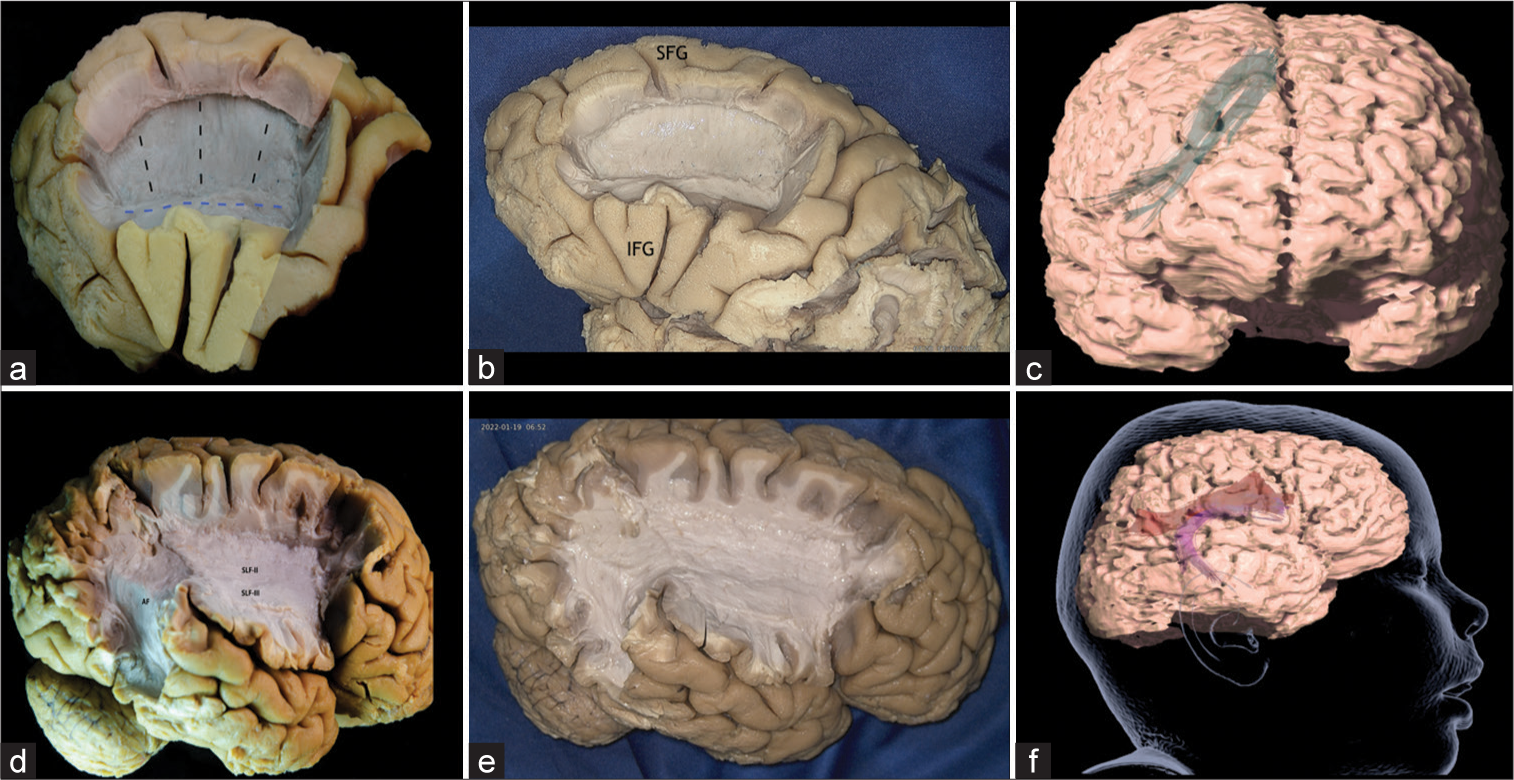
Case 3: Frontal aslant tract (FAT) fibers spread
Case 3 illustrates a typical example of a FAT epileptogenic activity spread from inferior frontal gyrus (IFG) to superior frontal gyrus (SFG). The FAT connects the supplementary motor area (SMA) and pre-SMA in the SFG to the pars opercularis and pars triangularis in the IFG. The FAT has been described as an oblique bundle of white matter fibers that emerge within the superolateral aspect of the SFG, curving gradually in the inferolateral direction (about 90°) to terminate into the IFG. The FAT runs medial to the superior longitudinal fasciculus (SLF) on the lateral aspect of the cortex[
Figure 4:
(a) Left frontal lobe after removal of the U-Fibers and part of SLF-II in the middle frontal gyrus, exposing the Aslant tract, which connects the supplementary motor area (SMA) and pre-SMA in the superior frontal gyrus to the pars opercularis and pars triangularis of the posterior inferior frontal gyrus. The inferior part of the SLF-II, which runs lateral to the aslant tract, is partially conserved. Yellow: primary focal cortical dysplasia epileptogenic zones (EZ), Red: ectopic EZ, Discontinuous black line: Aslant Tract, Discontinuous blue line: SLFII. (b) Left frontal lobe, exposing the Aslant tract. SFG: Superior frontal gyrus, IFG: Inferior frontal gyrus. (c) Tractography of the Aslant Tract. (d) Lateral view of the right hemisphere, after removal U-fibers of the middle and inferior frontal gyrus, inferior parietal lobe and the posterior part of the superior and middle temporal gyrus, exposing the SLF-II (originates in the angular gyrus and anterior intraparietal sulcus and terminates in the posterior regions of middle and superior frontal gyrus), SLF-III (joins the intraparietal sulcus and inferior parietal lobule to the inferior frontal gyrus), and the AF (connects Broca’s Area and Wernicke’s Area through frontal, parietal, and temporal Lobes). SLF-II: Superior longitudinal fascicle segment II, SLF-III: Superior longitudinal fascicle segment III, AF: Arcuate Fascicle. (e) Right frontal lobe, exposing the SLF-II, SLF-III, and the AF. (f) Tractography of the SLF-II and AF.
Case 4: SLF fibers spread
Case 4 illustrates SLF-II implication in spreading epileptogenic activity from a primary FCD lesion located in the left precentral/postcentral gyri (C3) and left angular gyrus (P3), to the MFG (F3). The long association fibers of the SLF connect frontal and parietal cortical regions in the human brain. The SLF comprises of three distinct sub-bundles, each presenting different specific functional roles and anatomical trajectories. The SLF-I connects the superior parietal lobule and precuneus to the SFG and anterior cingulate areas. The SLF-II originates in the angular gyrus and anterior intraparietal sulcus and terminates in the posterior regions of MFG and SFG. The SLF-III, however, joins the intraparietal sulcus and inferior parietal lobule to the IFG[
Case 5: Arcuate fascicle (AF) fibers spread
Epileptogenic spread through the AF from the primary FCD lesion epileptogenic foci located in the left superior and middle temporal gyrus (T3, T5), to the triangular part of the IFG (F7), was highlighted in Case 5. The AF is a peri-insular white matter tract that connects Broca’s Area and Wernicke’s Area through frontal, parietal, and temporal lobes[
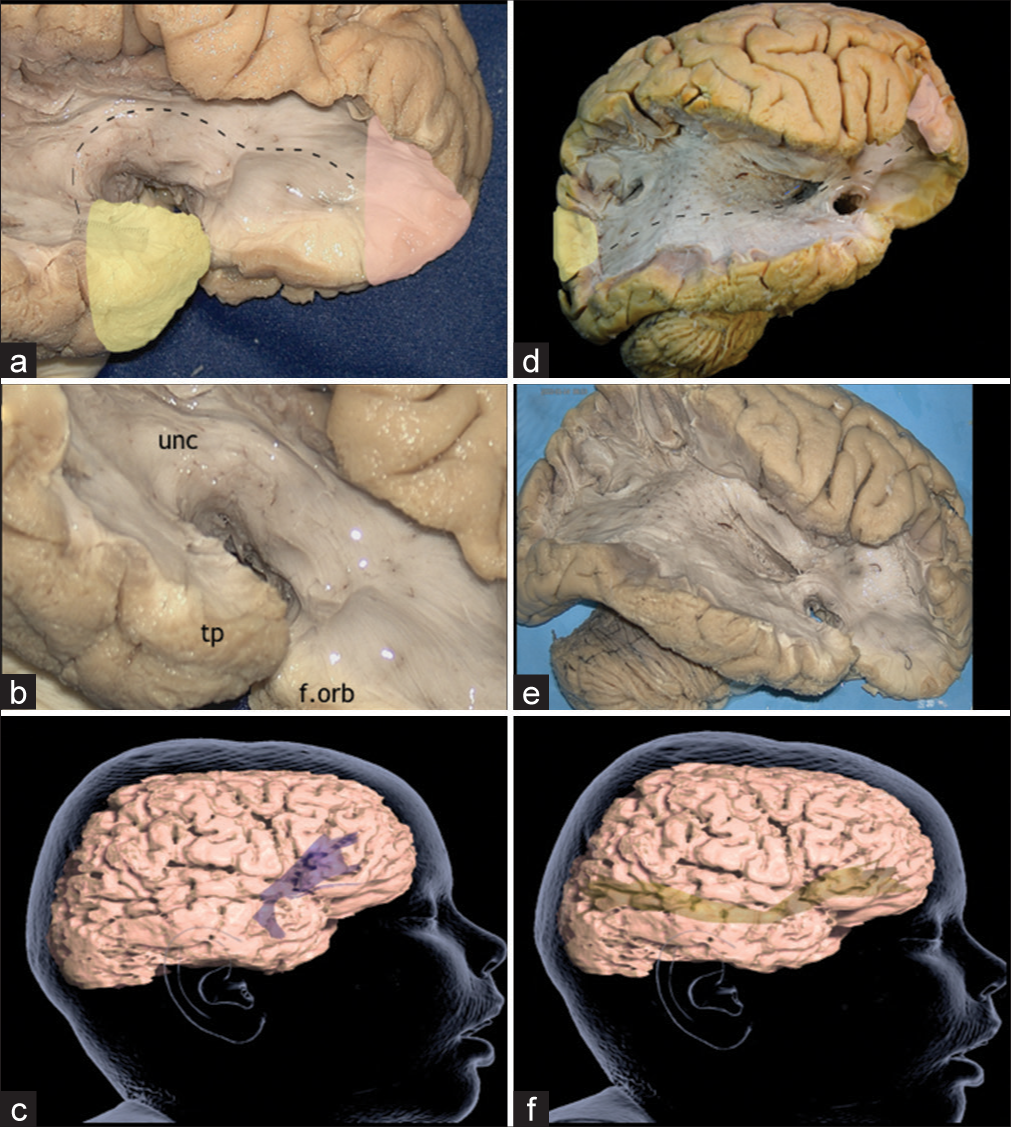
Case 6: Uncinate fasciculus(UF) spread
Primary epileptogenic foci (FCD) in the temporal lobe may produce excitable epileptogenic spread through the UF as illustrated in Case 6. The EEG in-depth epileptogenic spread was traced from the right medial temporal lobe to the right IFG (F8). The UF is a C-shaped white matter pathway that connects orbitofrontal cortex with the anterior temporal lobe. Although the UF cortical associations are still a matter of debate, it has been described that it connects with temporal and frontal poles, amygdala, hippocampus, orbital gyri, subcallosal area, gyrus rectus, IFG, and anterior temporal convexity.[
Figure 5:
(a) Lateral view of the right temporal and frontal poles, after exposing the uncinate fascicle, which connects the orbitofrontal cortex to the anterior temporal lobe. Yellow: primary focal cortical dysplasia epileptogenic zones (EZ), Red: ectopic EZ, Discontinuous line: Uncinate fascicle. (b) Lateral view of the right frontal and temporal lobes, after the dissection of the Uncinate Fascicle. tp: Temporal pole, unc: Uncinate Fascicle, f.orb: Orbitofrontal cortex. (c) Tractography of the uncinate fascicle. (d) Lateral view of the right hemisphere after dissection of U-fibers of the frontal, temporal, parietal, and occipital lobes, insula and part of the extreme capsule, exposing the inferior frontal occipital fasciculus (IFOF), which runs from the prefrontal area and orbitofrontal cortex directly to the parietal, occipital and postero-lateral temporal lobes. In the same picture, the right Uncinate Fascicle and Anterior Commissure can be appreciated. Yellow: primary focal cortical dysplasia EZ, Red: ectopic EZ, Discontinuous blue line: Anterior Commissure, Discontinuous black line: IFOF, Discontinuous white line: Uncinate fascicle. (e) Lateral view of the right hemisphere, exposing IFOF, uncinate fascicle, and anterior commissure. (f) Tractography of the IFOF.
Case 7: IFOF fibers spread
In Case 7, invasive EEG subdural electrodes depicted generalized discharges with a predominance of severity on the right occipital lobe (O2) with spread to the frontal lobe, suggesting an involvement of the IFOF. The IFOF is a long white matter associative tract that connects the prefrontal area and orbitofrontal cortex directly to the parietal, occipital, and posterolateral temporal lobes. The IFOF can be divided in 3 main segments: (1) a vertical segment that arises from the mid part of MFG and runs along the frontal lobe, (2) a horizontal segment that arises from pars orbitalis and triangularis of IFG and also runs along frontal lobe, and (3) a horizontal segment that courses from the limen insulae, travels into to the temporal stem and reaches the parietal and occipital lobes. IFOF, in the insular segment, composes the ventral part of the external capsule, while the dorsal part is composed by the claustrocortical fibers.[
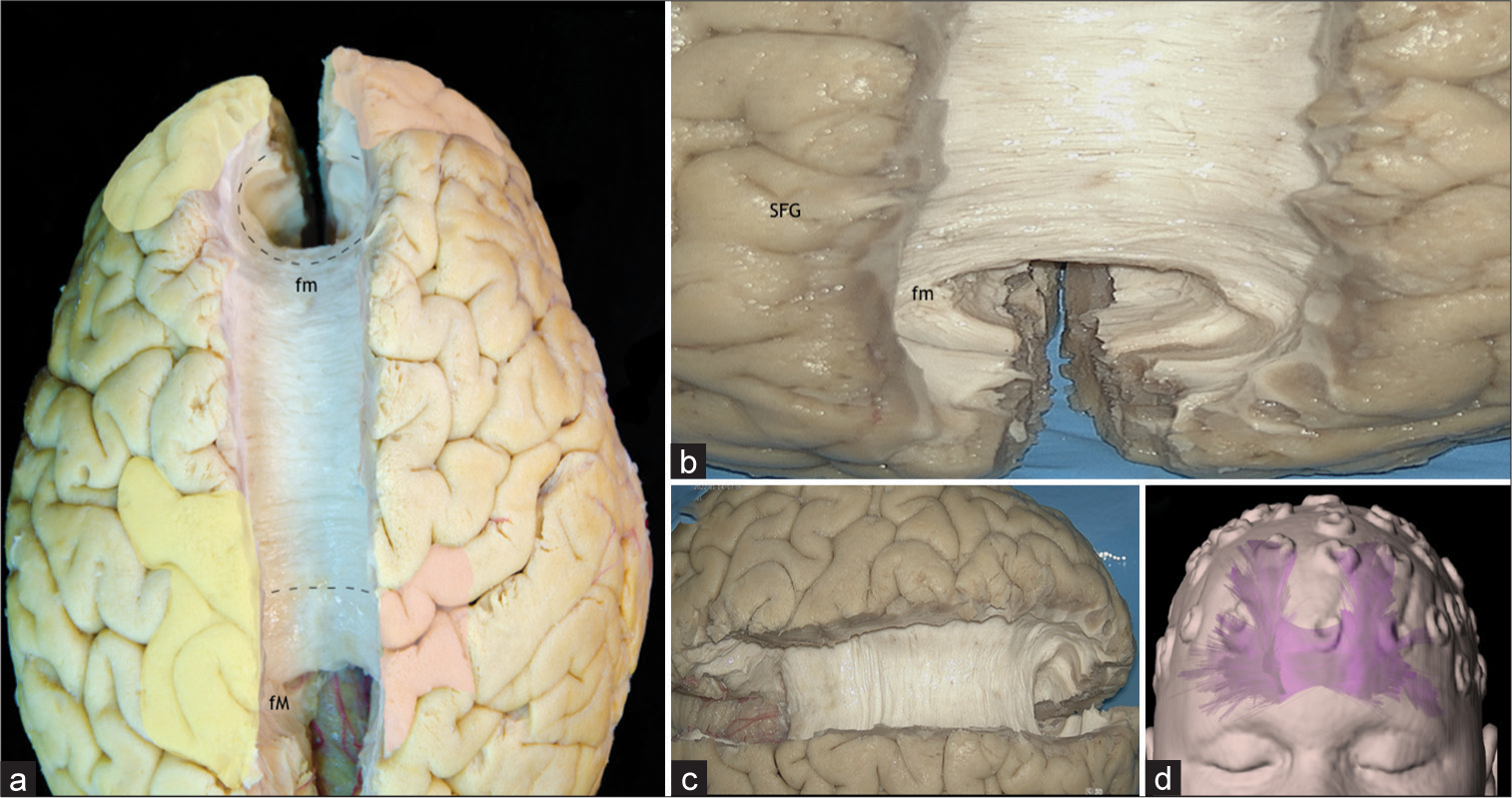
Cases 8 and 9: Corpus callosum (CC) fibers spread
In Case 8, EEG confirmed epileptogenic foci from the left frontal lobe (Fp1, F3), bilateral-synchronously, with epileptic foci in the right frontal lobe (Fp2, F4), suggesting spread through the forceps minor of the CC. In Case 9, an invasive stereotactic deep EEG reveled epileptogenic activity in the left and right parietal lobes (C3 and C4, respectively). Therefore, the spread to the contralateral parietal lobe was most likely mediated through the superior or dorsal radiations of the CC. The CC is the primary white matter commissural tract. Its pathway connects the occipital, temporal, parietal, frontal, limbic, insular lobes, and the basal ganglia of both hemispheres. Anatomically, from its anterior to posterior border, the CC is subdivided in five parts: rostrum, genu, body, isthmus, and splenium.[
Figure 6:
(a) Superior view of both hemispheres, after removal of the cingulum and the U-fibers of the medial surfaces, exposing the dorsal radiations of the corpus callosum (CC), the forceps major or posterior radiations and the forceps minor or anterior radiations. Yellow: Primary focal cortical dysplasia epileptogenic zones (EZ), Red: ectopic EZ, Discontinuous line: Commissural fibers of the CC, fm: Forceps Minor, fM: Forceps Major. (b) Superior view of both frontal lobes, after removal of the cingulum and the U-fibers of the medial surfaces, exposing the forceps minor or anterior radiations of the CC, which connects the medial surface and the basal region of both hemispheres. fm: Forceps minor, Superior frontal gyrus. (c) Superior view of both hemispheres, after removal of the cingulum and the U-fibers of the medial surfaces, exposing the dorsal radiations of the CC, the forceps major or posterior radiations and the forceps minor or anterior radiations. (d) Tractography of the CC. SFG: Superior frontal gyrus
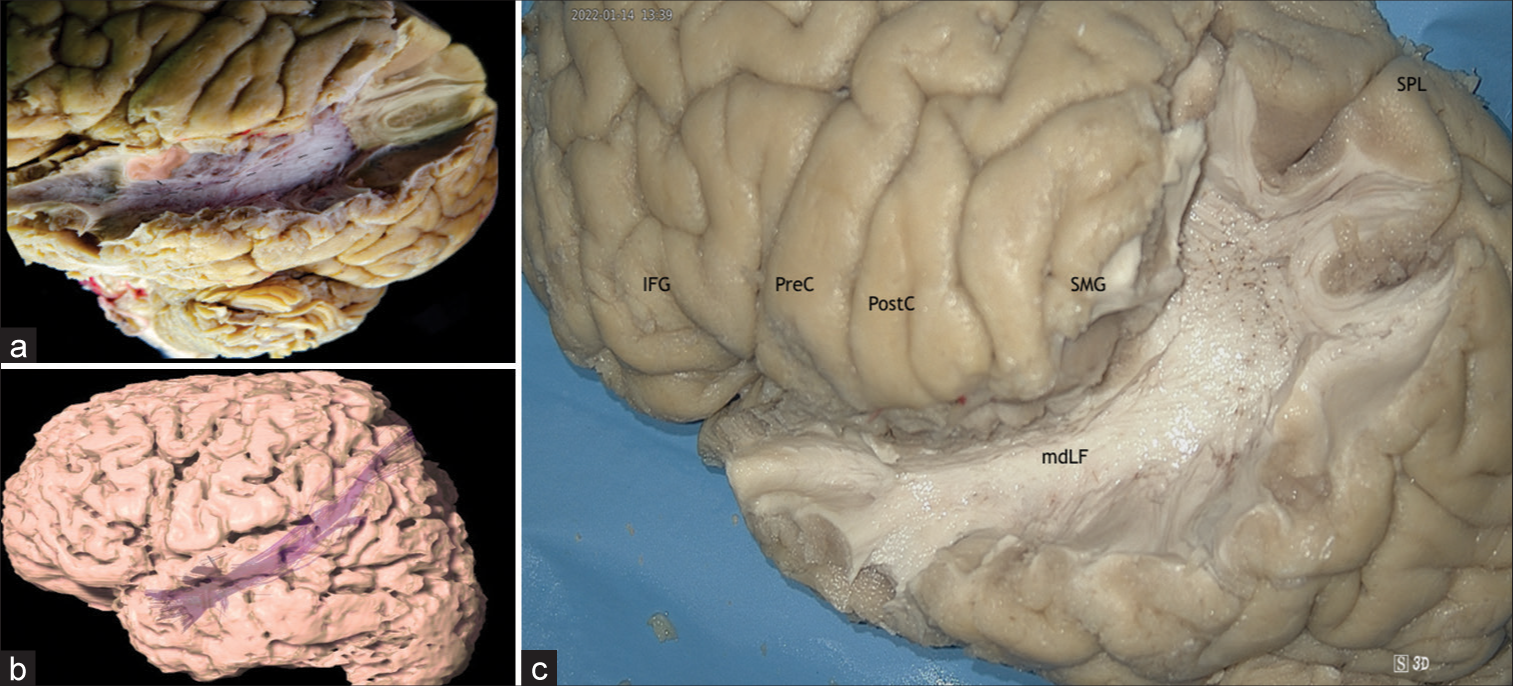
Case 10: Middle longitudinal fascicle (MdLF) fibers spread
In Case 10, invasive EEG considered a primary lesion in the superior temporal gyrus (STG) (T4), with suggestive spread to the postcentral and precentral gyri (C3), angular gyrus (P3) and the superior parietal lobe (Pz). Therefore, the FCD epileptogenic spread was somehow partially mediated through the MdLF. The MdLF is a long association pathway that runs in the lateral aspect of the brain and connects the STG with the parietal and occipital lobes. The MdLF travels through the anterior part of the STG and superior temporal sulcus area and under the U-fibers and the SLF/AF at the posterior temporal and inferior parietal lobes (anterolateral to posteromedial direction). It connects the STG to the superior parietal lobe and parietooccipital zone by running through the Transverse Gyri of Heschl; and the STG to the posterior area of the occipital cortex through the angular gyrus[
Figure 7:
(a) Lateral view of the left hemisphere after the removal of the U-fibers, superior longitudinal fasciculus, and arcuate fascicle, exposing the mdLF. The mdLF connects the superior temporal gyrus with the parietal and occipital lobes. Yellow: primary focal cortical dysplasia EZ; Red: ectopic EZ; Discontinuous line: mdLF. (b) Tractography of the mdLF. (c) Lateral view of the left hemisphere, after dissection of the mdLF. mdLF: Middle Longitudinal Fasciculus, SPL: Superior Parietal Lobe, IFG: Inferior frontal gyrus, PreC: Precental gyrus, PostC: Postcentral gyrus, SMG: Supramarginal Gyrus, EZ: Epileptogenic zones, and SLF: Superior longitudinal fasciculus.
DISCUSSION
Even though epilepsy surgery offers good postoperative seizure remission in FCD patients, adequate management of these cases is a daunting task.[
The epileptic zone and white matter tracts
Seizure networks generally start from the EZ which has within itself a SOZ. The SOZ can be the dysplastic area of the cortex from which clinical seizures actually arise.[
The symptomatogenic zone
The symptomatic zone produces the initial ictal symptoms.[
Moreover, some patients present symptomatology that could be related with the overexcitement or dysfunction of some white matter tracts.[
Surgery
To reduce the seizure burden and potentially improve cognitive function, a holistic and well encompassing FCD surgery must involve the total resection of the lesion, as well as its complete disconnection from the connecting fiber networks and EZ.[
The anterior temporal lobectomy followed by an amygdalohippocampectomy provided with a good postoperative outcome, especially in patients operated for FCD (II a – II b). We considered that FCD TLE afflicts not only the insulted cortex but also the white matter tracts within epileptogenic area, such as, the uncinate fascicle, the parahippocampal fibers, and the inferior frontolongitudinal fasciculus. After lateralizing the FCD, our limits of resection in the nondominant hemisphere extended 4–5 cm from the temporal pole on the STG and 3–5 cm in the language dominant one. Where we faced close typical speech eloquent zone lesion boundaries, multiple sub-pial transections were carried out to isolate the epileptic focus from the adjacent cortex.
The basis of performing a corpus callosotomy in our patient in Case 9 was generalized epileptogenic spread and drop attacks as highlighted in
It must be clear that even though we advocate for extensive and safe FCD lesion- EZ resection with the aim of achieving seizure control, white matter tracts function must be preserved. Where lesions are located near eloquent areas, functional monitoring is always highly recommended and even awake surgery could be considered in much selected cases.[
Future considerations
Recently, aspects for lesser invasive and effective methods of lesion resection have been taking center stage. Stereotactic laser ablation (SLA) for FCD offers promising prospective results, similar to open surgery cases.[
CONCLUSION
Understanding of the main implicated tracts in the epileptogenic spread in FCD patient remains cardinal for all those physicians dealing with epilepsy patients. To achieve seizure freedom, not only should the focal lesion be resected, but also its interconnections and tracts. It is therefore highly recommended that during EEG evaluation of the EZ and the ectopic foci, the main white matter tracts implicated are identified.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akeret K, Bellut D, Huppertz HJ, Ramantani G, König K, Serra C. Ultrasonographic features of focal cortical dysplasia and their relevance for epilepsy surgery. Neurosurg Focus. 2018. 45: E5
2. Altieri R, Melcarne A, Junemann C, Zeppa P, Zenga F, Garbossa D. Inferior Fronto-occipital fascicle anatomy in brain tumor surgeries: From anatomy lab to surgical theater. J Clin Neurosci. 2019. 68: 290-4
3. Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011. 77: 524-31
4. Barba C, Barbati G, Di Giuda D, Fuggetta F, Papacci F, Meglio M. Diagnostic yield and predictive value of provoked ictal SPECT in drug-resistant epilepsies. J Neurol. 2012. 259: 1613-22
5. Bartolini L, Whitehead MT, Ho CY, Sepeta LN, Oluigbo CO, Havens K. Temporal lobe epilepsy and focal cortical dysplasia in children: A tip to find the abnormality. Epilepsia. 2017. 58: 113-22
6. Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia. 2017. 58: 1131-47
7. Bast T, Ramantani G, Seitz A, Rating D. Focal cortical dysplasia: Prevalence, clinical presentation and epilepsy in children and adults. Acta Neurol Scand. 2006. 113: 72-81
8. Baynes K, Ramachandran VS, editors. Corpus callosum. Encyclopedia of the Human Brain. New York: Academic Press; 2002. p. 51-64
9. Briggs RG, Rahimi M, Conner AK, Sali G, Baker CM, Burks JD. A connectomic atlas of the human cerebrum-chapter 15: Tractographic description of the uncinate fasciculus. Oper Neurosurg (Hagerstown). 2018. 15: S450-55
10. Campos BM, Coan AC, Beltramini GC, Liu M, Yassuda CL, Ghizoni E. White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia. 2015. 56: 125-32
11. Choi SA, Kim SY, Kim H, Kim WJ, Kim H, Hwang H. Surgical outcome and predictive factors of epilepsy surgery in pediatric isolated focal cortical dysplasia. Epilepsy Res. 2018. 139: 54-9
12. Cobourn K, Fayed I, Keating RF, Oluigbo CO. Early outcomes of stereoelectroencephalography followed by MR-guided laser interstitial thermal therapy: A paradigm for minimally invasive epilepsy surgery. Neurosurg Focus. 2018. 45: E8
13. Cossu M, Fuschillo D, Bramerio M, Galli C, Gozzo F, Pelliccia V. Epilepsy surgery of focal cortical dysplasia-associated tumors. Epilepsia. 2013. 54: 115-22
14. Dick AS, Garic D, Graziano P, Tremblay P. The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex. 2019. 111: 148-63
15. Duchowny M. Clinical, functional, and neurophysiologic assessment of dysplastic cortical networks: Implications for cortical functioning and surgical management. Epilepsia. 2009. 50: 19-27
16. Fernández-Miranda JC, Rhoton AL, Alvarez-Linera J, Kakizawa Y, Choi C, de Oliveira EP. Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery. 2008. 62: 989-1026 discussion 26-8
17. Gharabaghi A, Kunath F, Erb M, Saur R, Heckl S, Tatagiba M. Perisylvian white matter connectivity in the human right hemisphere. BMC Neurosci. 2009. 10: 15
18. Gleichgerrcht E, Greenblatt AS, Kellermann TS, Rowland N, Vandergrift WA, Edwards J. Patterns of seizure spread in temporal lobe epilepsy are associated with distinct white matter tracts. Epilepsy Res. 2021. 171: 106571
19. Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: A systematic review. Epilepsia. 2016. 57: 1053-68
20. Guevara M, Guevara P, Román C, Mangin JF. Superficial white matter: A review on the dMRI analysis methods and applications. Neuroimage. 2020. 212: 116673
21. Herbet G, Zemmoura I, Duffau H. Functional anatomy of the inferior longitudinal fasciculus: From historical reports to current hypotheses. Front Neuroanat. 2018. 12: 77
22. Homan RW. The 10-20 Electrode System and Cerebral Location. Am J EEG Technol. 1988. 28: 269-79
23. Homan RW, Herman J, Purdy P. Cerebral location of international 10-20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987. 66: 376-82
24. Jobst BC, Kapur R, Barkley GL, Bazil CW, Berg MJ, Bergey GK. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017. 58: 1005-14
25. Jobst BC, Skarpaas TL, Morrell MJ. Response: Therapeutic brain-responsive neurostimulation in eloquent cortex can be delivered without symptoms. Epilepsia. 2017. 58: 1488
26. Kabdebon C, Leroy F, Simmonet H, Perrot M, Dubois J, Dehaene-Lambertz G. Anatomical correlations of the international 10-20 sensor placement system in infants. Neuroimage. 2014. 99: 342-56
27. Kadam R, Arimappamagan A, Rao MB, Sadashiva N, Mundlamuri RC, Raghavendra K. Posterior quadrant disconnection for childhood onset sub-hemispheric posterior head region epilepsy: Indications in an Indian cohort and outcome. Pediatr Neurosurg. 2021. 56: 538-48
28. Kadri PA, de Oliveira JG, Krayenbühl N, Türe U, de Oliveira EP, Al-Mefty O. Surgical approaches to the temporal horn: An anatomic analysis of white matter tract interruption. Oper Neurosurg (Hagerstown). 2017. 13: 258-70
29. Khoo HM, von Ellenrieder N, Zazubovits N, Hall JA, Dubeau F, Gotman J. Internodular functional connectivity in heterotopia-related epilepsy. Ann Clin Transl Neurol. 2019. 6: 1010-23
30. Kim DH, Kwon OY, Jung SW, Jeong H, Son S, Kim Sk. Location of irritative zone in epileptic brains of schizencephalic patients. Clin EEG Neurosci. 2016. 47: 235-42
31. La Corte E, Eldahaby D, Greco E, Aquino D, Bertolini G, Levi V. The frontal aslant tract: A systematic review for neurosurgical applications. Front Neurol. 2021. 12: 641586
32. Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo DT-MRI study. Cereb Cortex. 2005. 15: 854-69
33. Martino J, De Lucas EM. Subcortical anatomy of the lateral association fascicles of the brain: A review. Clin Anat. 2014. 27: 563-9
34. Martino J, De Witt Hamer PC, Vergani F, Brogna C, de Lucas EM, Vázquez-Barquero A. Cortex-sparing fiber dissection: An improved method for the study of white matter anatomy in the human brain. J Anat. 2011. 219: 531-41
35. Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping. Neuroimage. 2004. 21: 99-111
36. Palmini A. Electrophysiology of the focal cortical dysplasias. Epilepsia. 2010. 51: 23-6
37. Petito GT, Wharen RE, Feyissa AM, Grewal SS, Lucas JA, Tatum WO. The impact of stereotactic laser ablation at a typical epilepsy center. Epilepsy Behav. 2018. 78: 37-44
38. Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC. Altered white matter integrity in temporal lobe epilepsy: Association with cognitive and clinical profiles. Epilepsia. 2010. 51: 536-45
39. Santos MV, Machado HR. Extratemporal disconnective procedures for the treatment of epilepsy in children. Epilepsia. 2017. 58: 28-34
40. Shah A, Jhawar S, Goel A, Goel A. Corpus callosum and its connections: A fiber dissection study. World Neurosurg. 2021. 151: e1024-35
41. Shah A, Lunawat A, Jhawar SS, Goel A, Goel A. White fiber correlates of amygdalohippocampectomy through the middle temporal gyrus approach. World Neurosurg. 2022. 157: e156-65
42. Tringali G, Bono B, Dones I, Cordella R, Didato G, Villani F. Multimodal approach for radical excision of focal cortical dysplasia by combining advanced magnetic resonance imaging data to intraoperative ultrasound, electrocorticography, and cortical stimulation: A preliminary experience. World Neurosurg. 2018. 113: e738-46
43. Vavassori L, Sarubbo S, Petit L. Hodology of the superior longitudinal system of the human brain: A historical perspective, the current controversies, and a proposal. Brain Struct Funct. 2021. 226: 1363-84
44. Veersema TJ, Swampillai B, Ferrier CH, van Eijsden P, Gosselaar PH, van Rijen PC. Long-term seizure outcome after epilepsy surgery in patients with mild malformation of cortical development and focal cortical dysplasia. Epilepsia Open. 2019. 4: 170-5
45. Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. 2013. 136: 1692-707
46. Willie JT, Malcolm JG, Stern MA, Lowder LO, Neill SG, Cabaniss BT. Safety and effectiveness of stereotactic laser ablation for epileptogenic cerebral cavernous malformations. Epilepsia. 2019. 60: 220-32
47. Wirsich J, Perry A, Ridley B, Proix T, Golos M, Bénar C. Whole-brain analytic measures of network communication reveal increased structure-function correlation in right temporal lobe epilepsy. Neuroimage Clin. 2016. 11: 707-18
48. Yagmurlu K, Middlebrooks EH, Tanriover N, Rhoton AL. Fiber tracts of the dorsal language stream in the human brain. J Neurosurg. 2016. 124: 1396-405