- Departments of Neurosurgery, Ehime University School of Medicine, Shitsukawa, Toon, Ehime, Japan.
- Departments of Otolaryngology, Ehime University School of Medicine, Shitsukawa, Toon, Ehime, Japan.
- Division of Diagnostic Pathology, Ehime University School of Medicine, Shitsukawa, Toon, Ehime, Japan.
- Departments of Lifestyle-related Medicine and Endocrinology, Ehime University School of Medicine, Shitsukawa, Toon, Ehime, Japan.
Correspondence Address:
Akihiro Inoue
Departments of Neurosurgery, Ehime University School of Medicine, Shitsukawa, Toon, Ehime, Japan.
DOI:10.25259/SNI_111_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Akari Kusakawa, Akihiro Inoue, Yawara Nakamura, Naoya Nishida, Mana Fukushima, Hidenori Senba, Satoshi Suehiro, Shirabe Matsumoto, Masahiro Nishikawa, Saya Ozaki, Seiji Shigekawa, Hideaki Watanabe, Bunzo Matsuura, Riko Kitazawa, Takeharu Kunieda. Clinical features and endoscopic findings of granular cell tumor of the sellar region: A case report and review of the literature. 09-May-2020;11:101
How to cite this URL: Akari Kusakawa, Akihiro Inoue, Yawara Nakamura, Naoya Nishida, Mana Fukushima, Hidenori Senba, Satoshi Suehiro, Shirabe Matsumoto, Masahiro Nishikawa, Saya Ozaki, Seiji Shigekawa, Hideaki Watanabe, Bunzo Matsuura, Riko Kitazawa, Takeharu Kunieda. Clinical features and endoscopic findings of granular cell tumor of the sellar region: A case report and review of the literature. 09-May-2020;11:101. Available from: https://surgicalneurologyint.com/surgicalint-articles/10010/
Abstract
Background: Granular cell tumor (GCT) of the sellar region is a rare tumor of the sellar and suprasellar regions that originate from the neurohypophysis. This tumor is very difficult to differentiate from other pituitary neoplasms, such as pituitary adenoma, pituicytoma, and spindle cell oncocytoma. We report a rare case of GCT arising from the posterior pituitary of the sellar region and suggest a useful indicator for accurate diagnosis and pitfalls for surgical procedures.
Case Description: A 42-year-old woman was admitted to our hospital with bitemporal hemianopsia. Neuroimaging showed a large pituitary tumor in the sellar and suprasellar regions with a hypointense part on T2-weighted magnetic resonance imaging, and the enhanced anterior pituitary gland was displaced anteriorly. Laboratory findings showed mild hyperprolactinemia. Subtotal resection of the tumor was achieved using an endoscopic endonasal transsphenoidal approach. Histological findings showed round or polygonal cells with abundant granular eosinophilic cytoplasm staining strongly for thyroid transcription factor 1. The tumor was, therefore, diagnosed as a GCT of the sellar region, belonging to tumors of the posterior pituitary. After surgery, visual impairment and anterior pituitary function were improved. Follow-up neuroimaging after 1 year showed no signs of recurrence.
Conclusion: GCT of the sellar region is difficult to diagnose on routine neuroimaging. Therefore, accurate diagnosis requires careful identification of clinical signs, magnetic resonance imaging including hypointensity on T2-weighted imaging, and analysis of combined morphological and immunohistochemical studies.
Keywords: Granular cell tumor, Magnetic resonance imaging findings, Neurohypophysis, Pituicyte, Thyroid transcription factor 1
INTRODUCTION
Granular cell tumor (GCT) of the sellar region is a relatively rare neoplasm originating from the neurohypophysis, including the posterior pituitary and pituitary stalk/infundibulum.[
CASE DESCRIPTION
A 42-year-old woman presented to our department with slight visual deterioration [
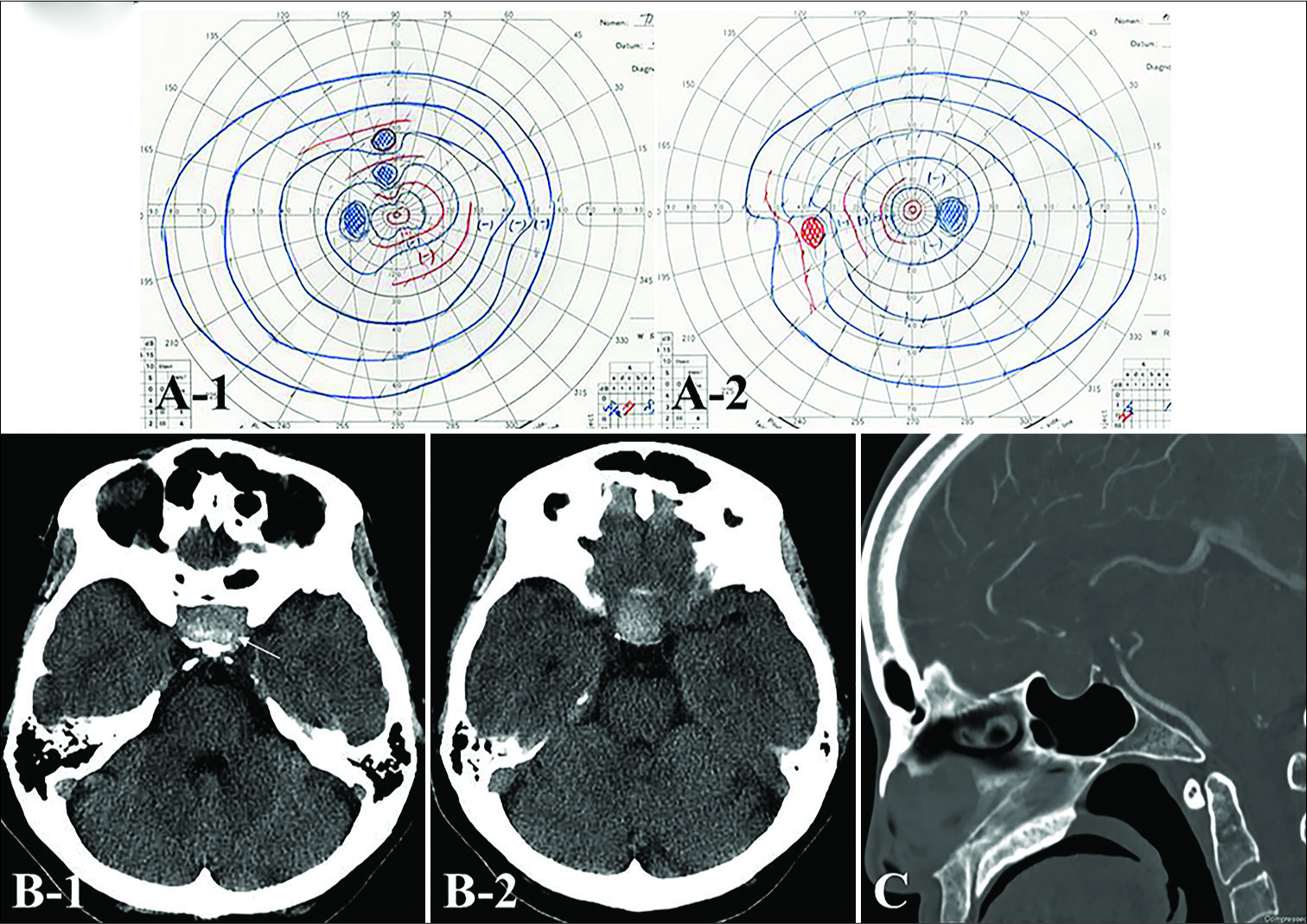
Figure 1:
Goldmann perimetry field examination on admission demonstrating a slight visual disturbance (A) Preoperative (B) axial and (C) sagittal computed tomography (CT) shows a high attenuated mass in the sellar region with granular-like high density dots (white arrow) accompanied by expansion of the sella turcica.
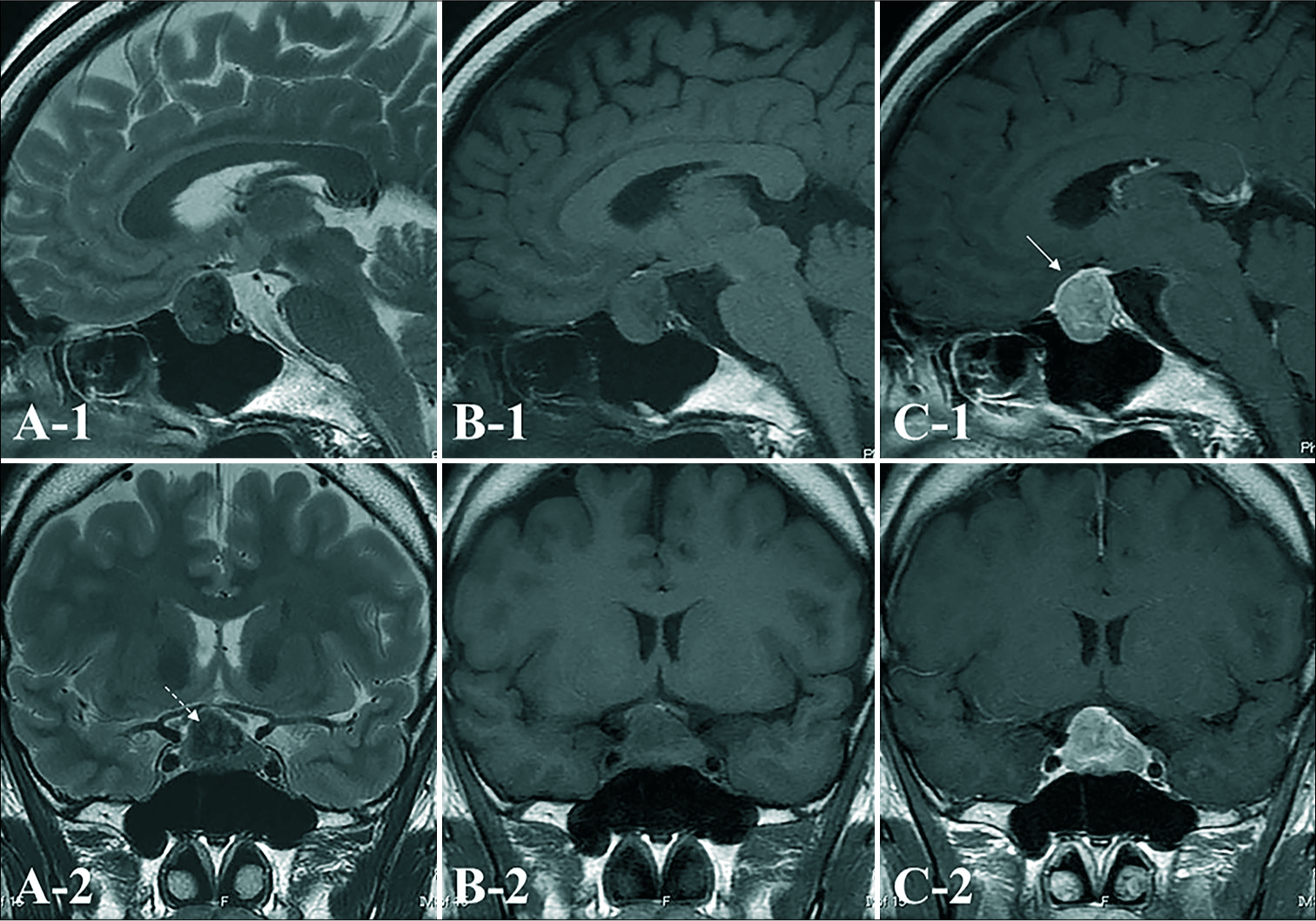
Figure 2:
On preoperative axial T2-weighted (A), T1-weighted (B), and gadolinium (Gd)-enhanced T1-weighted (C) magnetic resonance imaging (MRI), a tumor mass is seen in the sellar region extending into the suprasellar region. The tumor is homogeneously enhanced to a moderate degree with Gd. The enhanced anterior pituitary gland is displaced anteriorly (white arrow), and hyperintense on T1WI which suggested posteriorpituitary is appeared. The tumor is low intensity inside the solid mass on T2-weighted imaging (WI) (white dashed arrow).
Figure 3:
A) Intraoperative findings from endoscopic, endonasal, transsphenoidal surgery (PG: pituitary anterior gland; T: tumor). Macroscopic examination of this tumor shows that it is solid and rubbery-firm. The cut surface is yellowish, and uncountable gray to yellow granules are appear to be inside the solid mass. The tumor has infiltrated into the posterior pituitary; therefore, subtotal resection is performed. Postoperative (B-1) sagittal and (B-2) coronal images of Gd-enhanced MRI one year after surgical resection show no signs of recurrence
Figure 4:
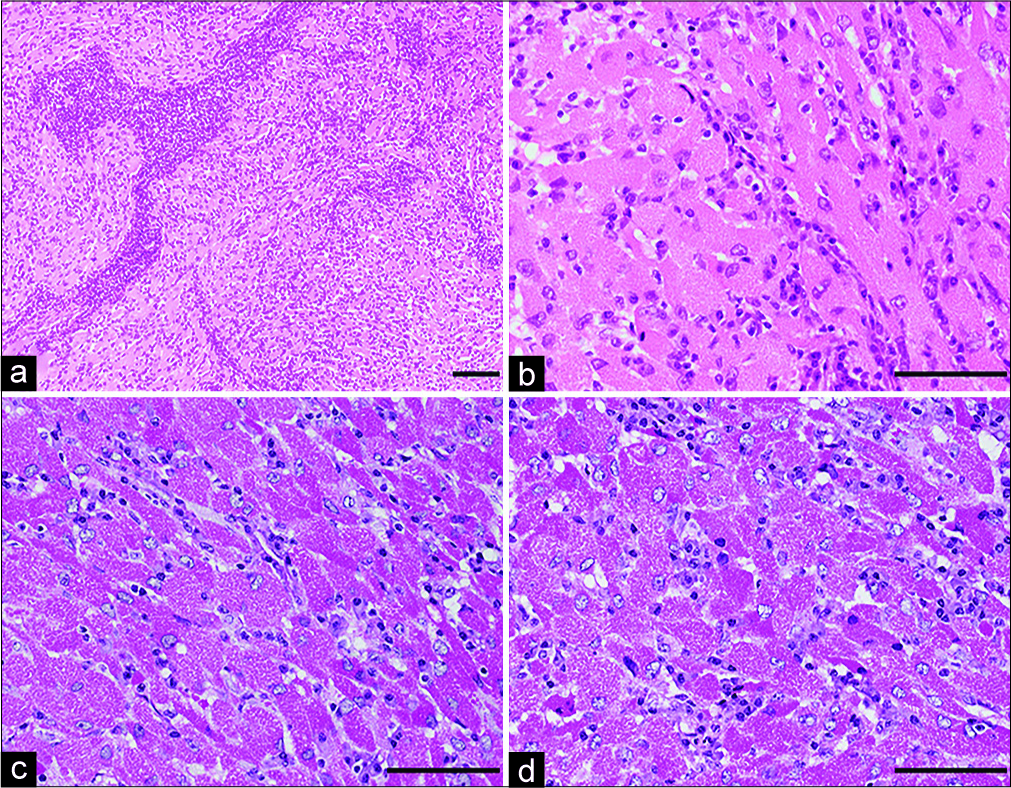
Histopathology of the resected tumor shows round or polygonal cells with abundant granular eosinophilic cytoplasm and perivascular lymphocytic aggregates. Most nuclei are round to oval in appearance without evidence of cellular atypia and mitotic figures (hematoxylin and e osin staining) (a, b). Periodic acid S chiff (PAS) staining of cytoplasmic granules is resistant to diastase digestion (c: PAS staining, d: diastaseresistant-positive PAS reaction). Magnification, a) ×100; b-d) ×400. Scale bar, a) 400 μm, b-d) 100 μm.
Figure 5:
Photomicrographs showing the histopathology of the tumor. Most tumor cells are immunoreactive for S-100 protein (a), but immune-negative for glial fibrillary acidic protein (GFAP) (b). This tumor shows slightly positive staining for Ki-67 (MIB-1) (MIB-1 labeling index: 2.0%) (c). In addition, almost all tumor cells are strongly positive for TTF-1 (d). Magnification, a, b, d) ×400; c) ×100. Scale bar, a, b, d) 400 μm, c) 100 μm.
DISCUSSION
GCT of the sellar region is a relatively rare primary tumor originating from the neurohypophysis, including the posterior pituitary and pituitary stalk/infundibulum.[
Clinically, this tumor often occurs in the sixth decade of life in men and the fifth decade in women and tends to develop slowly over a period of years, although patients can present with acute headache, confusion, diplopia, and visual disturbance.[
In the revised WHO classification of CNS tumors (2016), the definition of GCT of the sellar region is as follows: “A circumscribed tumor that is composed of large epithelioid to spindled cells with distinctively granular, eosinophilic cytoplasm and that arises from the neurohypophysis or infundibulum.” Microscopically, GCT consists mainly of densely packed polygonal cells with abundant granular eosinophilic cytoplasm, which was confirmed as lysosomes on electron microscopy, apparently different from neuroendocrine granules. The nuclei are a small round shape with little pleomorphism, and mitotic figures are rare.[
Finally, regarding the treatment of GCT of the sellar region, surgical resection has the most important role.[
CONCLUSION
In our view, GCT of the sellar region should be included as an important differential diagnosis for pituitary tumors due to the potential for very strong attachment to normal structures and the difficulty of surgical resection, unlike common pituitary tumors. Therefore, careful identification of the clinical signs and MRI findings and detailed evaluation of immunohistochemical studies are necessary for accurate diagnosis and appropriate treatment selection for GCT of the sellar region.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aki T, Inoue A, Kohno S, Nishida N, Shin Y, Fukushima M. Clinical features and endoscopic findings of pituicytoma in the sellar region: A case report and review of the literature. Interdiscip Neurosurg. 2019. 16: 58-61
2. Aquilina K, Kamel M, Kalimuthu SG, Marks JC, Keohane C. Granular cell tumour of the neurohypophysis: A rare sellar tumour with specific radiological and operative features. Br J Neurosurg. 2006. 20: 51-4
3. Becker DH, Wilson CB. Symptomatic parasellar granular cell tumors. Neurosurgery. 1981. 8: 173-80
4. Boyce R, Beadles C. A further contribution to the study of the pathology of the hypophysis cerebri. J Pathol Bacteriol. 1893. 1: 359-83
5. Christopher PR, Kingsley PA, Bedi HS, Kwatra KS, Rathore S, Das KC. Large mid-esophageal granular cell tumor: Benign versus malignant. Rare Tumors. 2015. 7: 5772-
6. Covington MF, Chin SS, Osborn AG. Pituicytoma, spindle cell oncocytoma, and granular cell tumor: Clarification and meta-analysis of the world literature since 1893. AJNR Am J Neuroradiol. 2011. 32: 2067-72
7. Han F, Gao L, Wang Y, Jin Y, Lv Y, Yao Z. Clinical and imaging features of granular cell tumor of the neurohypophysis: A retrospective analysis. Medicine (Baltimore). 2018. 97: e9745-
8. Hasegawa H, Kitano M, Yamashita S, Kugai M, Furube M, Tominaga S. A case of suprasellar granular cell tumor. Prog Neurol Oncol. 2016. 23: 25-30
9. Kasashima S, Oda Y, Nozaki J, Shirasaki M, Nakanishi I. A case of atypical granular cell tumor of the neurohypophysis. Pathol Int. 2000. 50: 568-73
10. Liu HL, Huang BY, Zhang MS, Wang HR, Qu YM, Yu CJ. Sellar and suprasellar granular cell tumor of neurohypophysis. Chin Med J (Engl). 2017. 130: 741-3
11. Losa M, Saeger W, Mortini P, Pandolfi C, Terreni MR, Taccagni G. Acromegaly associated with a granular cell tumor of the neurohypophysis: A clinical and histological study. Case report. J Neurosurg. 2000. 93: 121-6
12. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
13. Luthy F, Klingler M. The tumoret tumor of the posterior pituitary lobe. Schweiz Z Pathol Bakteriol. 1951. 14: 721-9
14. Yang LJ, Huang XY, Han GX, Shen XD, Mu YM, Li TS. Ectopic thyroid masquerading as pituitary adenoma. Chin Med J (Engl). 2015. 128: 3389-90