- Department of Neurosurgery, Tokyo Women’s Medical University Adachi Medical Center,
- Department of Dermatology, Tokyo Women’s Medical University Adachi Medical Center,
- Department of Neurosurgery, Neuro Machida Clinic, Tokyo, Japan.
Correspondence Address:
Asami Kikuchi, Department of Neurosurgery, Tokyo Women’s Medical University Adachi Medical Center, 4-33-1 Kouhoku, Adachi-Ku, Tokyo, 123-8558, Japan.
DOI:10.25259/SNI_544_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Asami Kikuchi1, Sumiko Ishizaki2, Suguru Yokosako1, Hidetoshi Kasuya3, Yuichi Kubota1. Clinical features of herpes simplex virus reactivation after microvascular decompression for trigeminal neuralgia: Experience of 200 patients and a literature review. 19-Aug-2022;13:371
How to cite this URL: Asami Kikuchi1, Sumiko Ishizaki2, Suguru Yokosako1, Hidetoshi Kasuya3, Yuichi Kubota1. Clinical features of herpes simplex virus reactivation after microvascular decompression for trigeminal neuralgia: Experience of 200 patients and a literature review. 19-Aug-2022;13:371. Available from: https://surgicalneurologyint.com/surgicalint-articles/11805/
Abstract
Background: Herpes simplex virus (HSV) reactivation occasionally develops in the early postoperative period after microvascular decompression (MVD) for trigeminal neuralgia (TN). Therefore, the present study investigated the clinical features of this phenomenon.
Methods: The study cohort comprised 200 patients with 125 women aged between 17 and 90 years (median age, 66 years) who underwent MVD for TN between January 2010 and December 2020. Characteristics were compared between patients with and without HSV reactivation and clinical features were analyzed.
Results: Twenty patients had HSV reactivation: herpes labialis in 18 and herpes zoster (final diagnosis) in 2. A multivariate analysis revealed independent correlations between postoperative HV reactivation and a previous history of herpes labialis (odds ratios [OR]: 6.32, P = 0.0003) and reoperation for recurrent or persistent pain (OR: 5.06, P = 0.0211). No significant differences were observed in pain relief, postoperative facial numbness, or Barrow Neurological Institute Pain Intensity/Facial Numbness Scores in the past follow-up between patients with and without HSV reactivation. HSV reactivation manifested at a median of the 4th postoperative day (1–10 days) and its location was not related to the preoperative distribution of facial pain. All patients were treated with local acyclovir and were completely cured within 1–2 weeks.
Conclusion: HSV reactivation occurred in 10% of patients after MVD including 1% of herpes zoster. A previous history of herpes labialis and reoperation was identified as risk factors for reactivation. Symptoms were completely cured by antiviral drugs within 1–2 weeks. It is important to note that cases of herpes zoster may be confused with cases of HSV after MVD.
Keywords: Herpes simplex, Herpes zoster, Microvascular decompression, Reactivation, Trigeminal neuralgia
INTRODUCTION
Burdick et al.[
MATERIALS AND METHODS
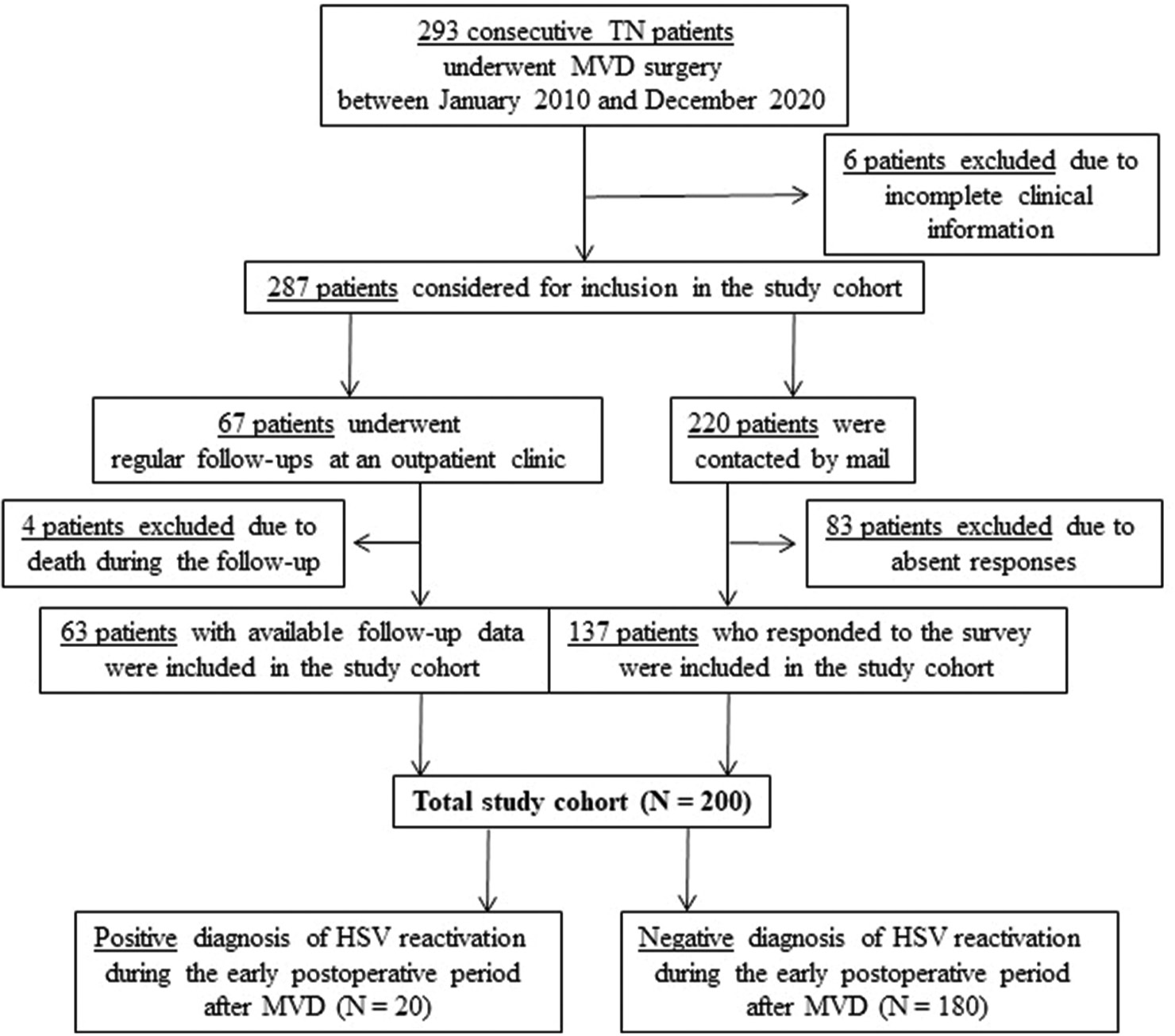
Between January 2010 and December 2020, 293 consecutive patients with TN underwent MVD in the Tokyo Women’s Medical University Medical Center East (currently Adachi Medical Center).
All surgeries were performed by the senior author (H.K.) after patients had provided their informed consent, as previously reported.[
Pain relief and facial numbness were assessed according to the Barrow Neurological Institute (BNI) Pain Intensity and Facial Numbness Score.[
Statistical analysis
Subgroup comparisons were performed using Fisher’s exact test and the Wilcoxon signed-rank sum test for categorical and continuous variables, respectively. The level of significance was defined as P < 0.05. Factors demonstrating significant or borderline (P < 0.1) relationships with the variable of interest (“postoperative HSV reactivation”) in the univariate analysis were included in multivariate modeling using logistic regression and odds ratios (OR) and their 95% confidence intervals (CI). All calculations were performed using the free software R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
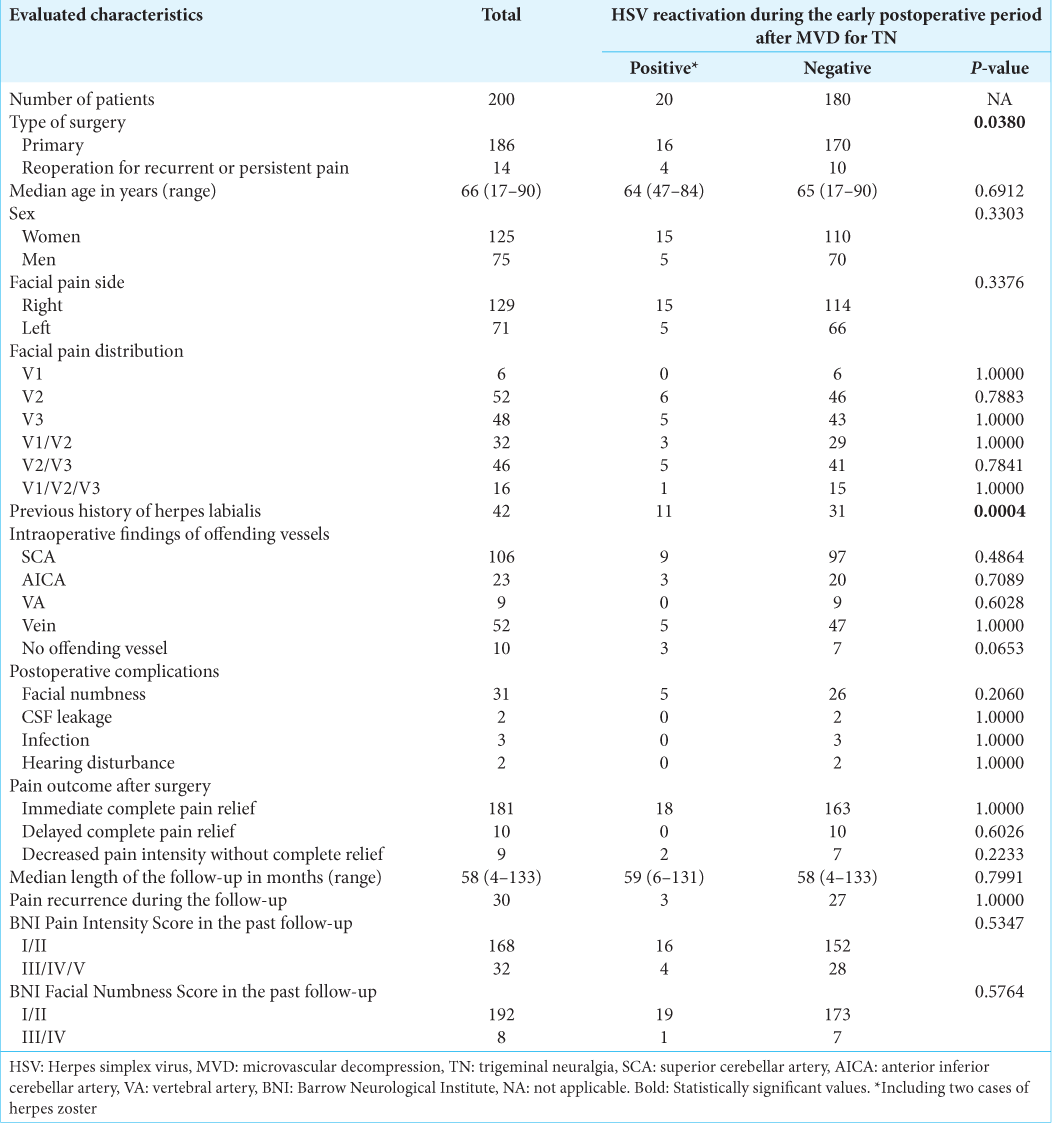
The characteristics of patients in the study cohort are shown in
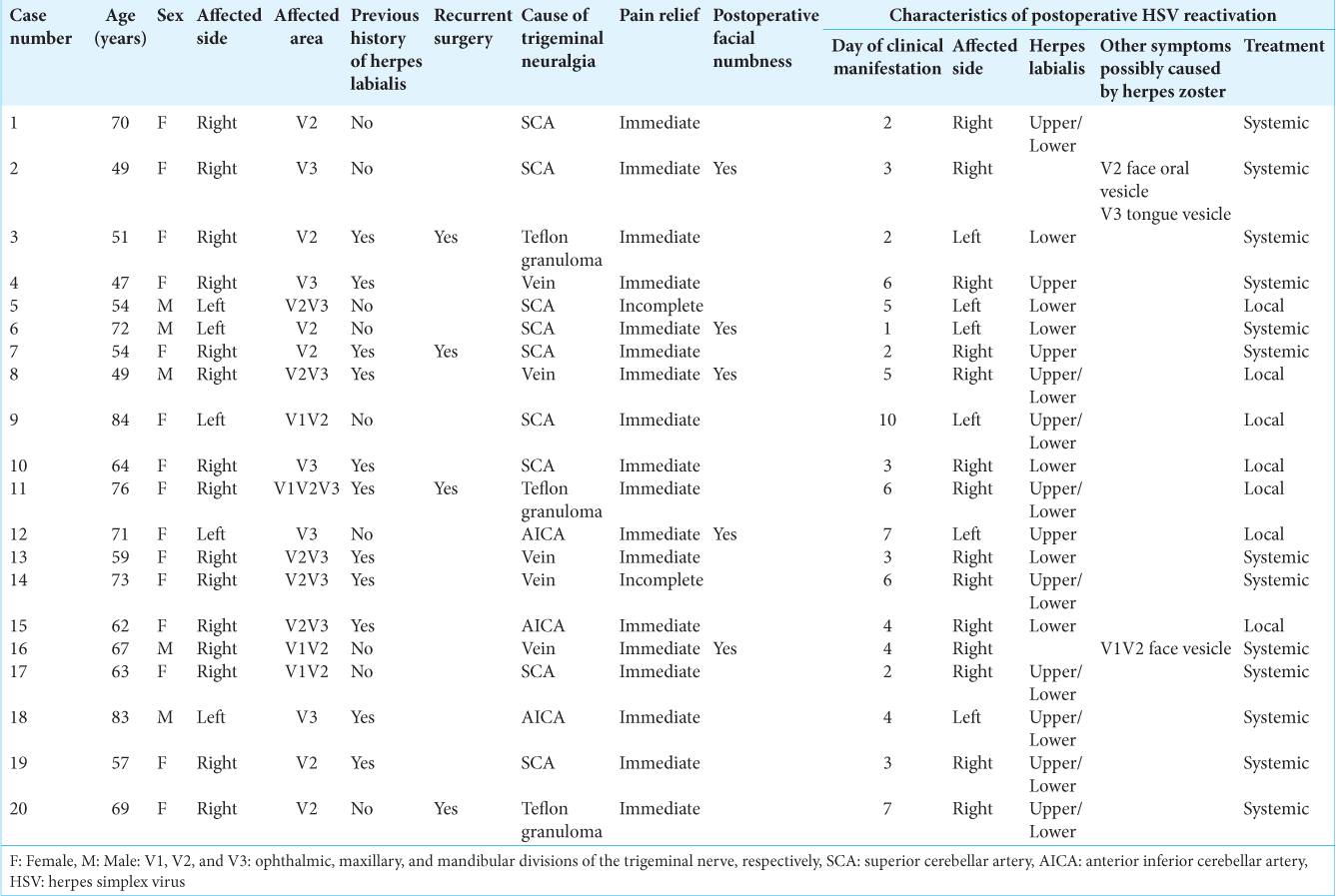
Among 200 patients with TN after MVD, the reactivation of HSV was detected in 20 patients: 18 with herpes labialis, one with face, oral, and tongue vesicles, and one with face vesicles. Dermatologists finally diagnosed the latter two as herpes zoster [
Comparisons of patients with and without a positive diagnosis of HV reactivation during the early postoperative period after MVD revealed significantly higher rates of reoperation (20% vs. 6%, P = 0.0380) and a previous history of herpes labialis (55% vs. 17%, P = 0.0004). In addition, the absence of the intraoperatively identified offending vessel was more frequently noted (P = 0.0653) in patients with postoperative HSV reactivation. An analysis of these three predictive factors in the multivariate model revealed independent correlations between HSV reactivation and a previous history of herpes labialis (OR: 6.32, 95% CI: 2.34–17.08, P = 0.0003) and reoperation (OR: 5.06, 95% CI: 1.28–20.06, P = 0.0211). None of the other factors evaluated significantly differed between the subgroups. Furthermore, no significant differences were observed in pain relief, postoperative facial numbness, or BNI Pain Intensity/Facial Numbness Scores at the last follow-up between patients with and without HSV reactivation [
Symptoms appeared at a median of the 4th postoperative day (1–10 days). One patient exhibited symptoms on the opposite site, whereas ten patients developed herpes labialis elsewhere to the division of neuralgia [
DISCUSSION
HSV reactivation occurred in 10% of patients after MVD for TN, including 1% with herpes zoster, and a herpes history of herpes labialis and reoperation was identified as risk factors for reactivation. The most common symptoms are herpes labialis, with 3–5 vesicles on the vermillion border of the lip preceded by pain, while the incidence of herpes simplex encephalitis following neurosurgery due to virus reactivation is very low.[
When HV reactivation occurs in patients with atopic dermatitis or those in an immunocompromised state, it may have a severe clinical presentation called Kaposi’s varicelliform eruption, for which the head, neck, and trunk are the predominant sites. Complications include keratoconjunctivitis, meningitis, and encephalitis.[
Patients in the present study were treated with systemic and local antivirus drugs and herpes labialis was completely cured within 1–2 weeks without deterioration. Oral acyclovir reduces the time to the loss of crusts from approximately 7 to 8 days, but does not alter the time required for complete healing.[
Herpes zoster is included in HSV reactivation after MVD. Cushing,[
Varicella-zoster virus is a pathogenic human herpes virus that causes varicella (chickenpox) as a primary infection, following which it becomes latent in peripheral ganglia. Decades later, the virus may reactivate spontaneously or after a number of triggering factors to cause herpes zoster (shingles).[
HSV reactivation did not always occur at the area of facial pain or on the side of the manipulation. Since the whole trigeminal root is manipulated in MVD, the area of HSV reactivation is not always the same division of the trigeminal nerve. We, previously, encountered one patient who developed symptoms on the other side of surgery.[
HSV reactivation occurred in 10% of patients after MVD for TN, including 1% of herpes zoster, and a herpes history of herpes labialis and reoperation was identified as risk factors for reactivation. HSV reactivation occurs in infected HSV patients with a history of herpes labialis, although primary infections are commonly asymptomatic.[
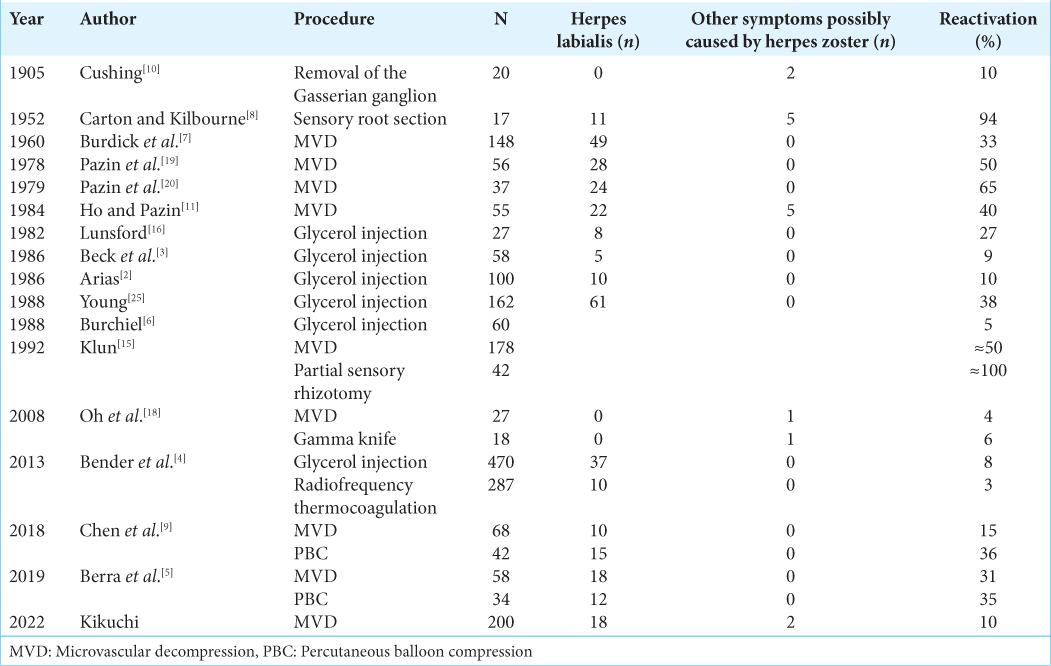
The incidence of HSV reactivation after various procedures on the trigeminal nerve in patients with TN is shown in
CONCLUSION
HSV reactivation occurred in 10% of patients after MVD including 1% of herpes zoster. A previous history of herpes labialis and reoperation was identified as risk factors for reactivation. A minimal stimulation or inapparent trauma to the trigeminal sensory root is sufficient to activate latent herpes virus. Symptoms were mild and cured by normal treatment without severe complications. It is important to note that cases of herpes zoster may be confused with those of HSV.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are thankful to Drs. Shigeru Tani and Mikhail Chernov for their help during study and preparing of this paper for publication.
References
1. Arduino PG, Porter SR. Herpes simplex virus Type 1 infection: Overview on relevant clinico-pathological features. J Oral Pathol Med. 2008. 37: 107-21
2. Arias MJ. Percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia. A prospective study of 100 cases. J Neurosurg. 1986. 65: 32-6
3. Beck DW, Olson JJ, Urig EJ. Percutaneous retrogasserian glycerol rhizotomy for treatment of trigeminal neuralgia. J Neurosurg. 1986. 65: 28-31
4. Bender MT, Pradilla G, Batra S, See AP, James C, Pardo CA. Glycerol rhizotomy and radiofrequency thermocoagulation for trigeminal neuralgia in multiple sclerosis. J Neurosurg. 2018. 118: 329-36
5. Berra LV, Armocida D, Pesce A, Di Rita A, Santoro A. Herpes simplex reactivation after surgical treatment of trigeminal neuralgia: A retrospective cohort study. World Neurosurg. 2019. 127: e16-21
6. Burchiel KJ. Percutaneous retrogasserian glycerol rhizolysis in the management of trigeminal neuralgia. J Neurosurg. 1988. 69: 361-6
7. Burdick KH, Haserick JR, Gardner WJ. Herpes simplex following decompression operations for trigeminal neuralgia. Attempts to modify by local use of hydrocortisone preparations. Arch Dermatol. 1960. 81: 919-21
8. Carton CA, Kilbourne ED. Activation of latent herpes simplex by trigeminal sensory-root section. N Engl J Med. 1952. 246: 172-6
9. Chen JN, Yu WH, Du HG, Jiang L, Dong XQ, Cao J. Prospective comparison of redo microvascular decompression and percutaneous balloon compression as primary surgery for recurrent trigeminal neuralgia. J Korean Neurosurg Soc. 2018. 61: 747-52
10. Cushing H. The surgical aspects of major neuralgia of the trigeminal nerve: A report of twenty cases of operation on the Gasserian ganglion with anatomic and physiologic notes on the consequences of its removal. JAMA. 1905. 44: 1002-8
11. Ho M, Pazin GJ, Armstrong JA, Haverkos HS, Dummer JS, Jannetta PJ. Paradoxical effects of interferon on reactivation of oral infection with herpes simplex virus after microvascular decompression for trigeminal neuralgia. J Infect Dis. 1984. 150: 867-72
12. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017. 31: 811-26
13. Kasuya H, Tani S, Kubota Y, Yokosako S, Ohbuchi H, Arai N. Characteristics and management of the offending veins in microvascular decompression surgery for trigeminal neuralgia. Neurosurg Rev. 2021. 44: 2337-47
14. Kennedy PG, Gershon AA. Clinical features of varicella-zoster virus infection. Viruses. 2018. 10: 609
15. Klun B. Microvascular decompression and partial sensory rhizotomy in the treatment of trigeminal neuralgia: Personal experience with 220 patients. Neurosurgery. 1992. 30: 49-52
16. Lunsford LD. Treatment of tic douloureaux by percutaneous retrogasserian glycerol injection. JAMA. 1982. 248: 449-53
17. McLaughlin DC, Achey RL, Geertman R, Grossman J. Herpes simplex reactivation following neurosurgery: Case report and review of the literature. Neurosurg Focus. 2019. 47: E9
18. Oh IH, Choi SK, Park BJ, Kim TS, Rhee BA, Lim YJ. The treatment outcome of elderly patients with idiopathic trigeminal neuralgia: Micro-vascular decompression versus gamma knife radiosurgery. J Korean Neurosurg Soc. 2008. 44: 199-204
19. Pazin GJ, Ho M, Jannetta PJ. Reactivation of herpes simplex virus after decompression of the trigeminal nerve root. J Infect Dis. 1978. 138: 405-9
20. Pazin GJ, Armstrong JA, Lam MT, Tarr GC, Jannetta PJ, Ho M. Prevention of reactivated herpes simplex infection by human leukocyte interferon after operation on the trigeminal root. N Engl J Med. 1979. 301: 225-30
21. Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: The initial experience of the barrow neurological institute. Int J Radiat Oncol Biol Phys. 2000. 47: 1013-9
22. Simms HN, Dunn LT. Herpes zoster of the trigeminal nerve following microvascular decompression. Br J Neurosurg. 2006. 20: 423-6
23. Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001. 357: 1513-8
24. Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ. Trends in herpes simplex virus Type 1 and Type 2 seroprevalence in the United States. JAMA. 2006. 296: 964-73
25. Young RF. Glycerol rhizolysis for treatment of trigeminal neuralgia. J Neurosurg. 1988. 69: 39-45