- Department of Neurosurgery, Institute of Medical Sciences, Banaras Hindu University, Lanka, Varanasi, Uttar Pradesh, India.
DOI:10.25259/SNI_128_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rahul Singh, Anurag Sahu, Kulwant Singh, Ravi Shankar Prasad, Nityanand Pandey. Clinical, operative, and outcome analysis of giant extradural hematoma: A retrospective study in tertiary care center. 08-Aug-2020;11:236
How to cite this URL: Rahul Singh, Anurag Sahu, Kulwant Singh, Ravi Shankar Prasad, Nityanand Pandey. Clinical, operative, and outcome analysis of giant extradural hematoma: A retrospective study in tertiary care center. 08-Aug-2020;11:236. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10190
Abstract
Background: This study is aimed to find a critical volume of operated giant or massive extradural hematoma (EDH) that affects outcome significantly and analyze them with respect to their clinical, surgical, and outcome perspective.
Methods: This retrospective study includes 253 patients operated for EDH in emergency in the Department of Neurosurgery of IMS BHU, Varanasi, India, a tertiary care center, between August 1, 2018, and November 1, 2019. Giant EDH critical volume was evaluated. Twenty-nine patients with giant EDH with clot volume ≥ 80 ml were further analyzed for clinical, surgical, and outcome predictive factors. Statistical analysis was done using Prism GraphPad ver. 8.0.0. P value was taken at 0.05.
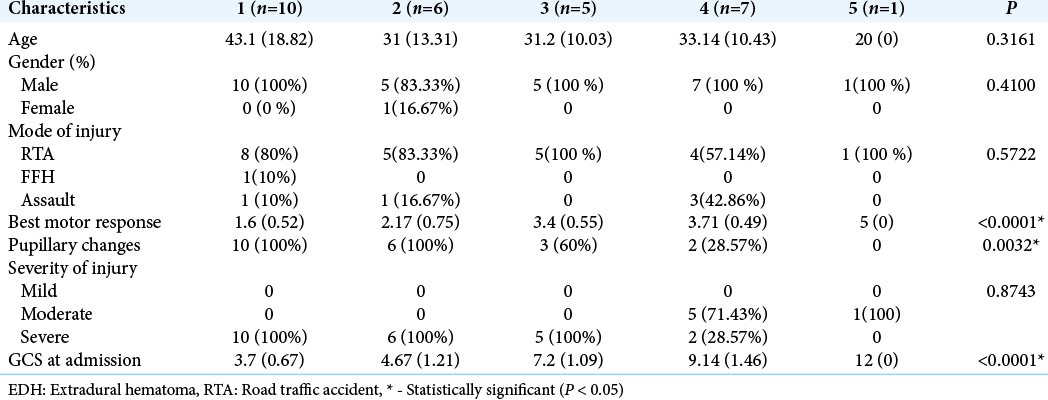
Results: Dichotomized group analysis with Glasgow Outcome Score (GOS) 4–5 versus GOS 1–3 for testing clot volume revealed significance difference with P 80 ml for further analysis. The most common age group was 20–40 (55.17%). M2 (31.03%) was the most common best motor response in operated giant EDH cases. Most of them were having severe (79.31%) head injury. Glasgow Coma Scale (GCS) at admission (P P = 0.0032), and best motor response (P P
Conclusion: Giant EDH with volume ≥ 80 ml is associated with poorer outcome. GCS at admission, pupillary changes, and best motor response is predictors for surgical outcome of giant EDH.
Keywords: Best motor response, Extradural hematoma, Giant extradural hematoma, Glasgow outcome score, Massive extradural hematoma
INTRODUCTION
Extradural hematoma (EDH) is a collection of blood clots between the outer layer of dura and inner table of skull. It is usually caused due to rupture of middle meningeal artery, but can also be caused by ruptured dural venous sinuses, diploic veins, meningeal vein, or fracture line bleeding. Its origin is almost always traumatic and usually associated with a skull fracture.[
CT scan is the investigation of choice to detect intracranial injury after trauma.[
The associated brain damage is mainly caused by local ischemia due to mass effect or direct brain injury or hampered venous outflow. Ischemic cerebral damage is a very important prognostic factor in the pathology of EDH and may be due to mass effects of the hematoma and raised intracranial pressure (ICP), leading to compromised cerebral perfusion pressure (CPP).
The accompanying brain damage beside EDH is responsible for poor neurological function after injury. Outcome of EDH depends most importantly on preoperative Glasgow Coma Scale (GCS) and neurological status.[
Giant or massive EDH [
Operative outcome of EDH depends on multitude of factors, namely, multiple comorbidities, coexisting brain injury, cerebral ischemia, antithrombotic therapy, and geriatric age group. A correct identification of outcome predictive factors is crucial for appropriate neurosurgical intervention. In this study, we evaluated a critical volume of giant or massive EDH that causes enough mass effect leading to significant immediate deterioration of neurological status and resultant poor outcome. It is also aimed at analyzing operated giant EDH cases with respect to their clinical, operative, and outcome perspective. There is a paucity of literature review over determination of critical volume of giant EDH.
MATERIALS AND METHODS
This retrospective observational study includes analysis of 253 patients operated for EDH on emergency basis in the Department of Neurosurgery of IMS BHU, Varanasi, India, a tertiary care center, between August 1, 2018, and November 1, 2019. Analysis of 253 patients was done for the evaluation of giant EDH critical volume. Based on this analysis, 29 patients with giant EDH with clot volume ≥80 ml were further analyzed for clinical, surgical, and outcome. The outcome of 253 operated cases of EDH with respect to clot volume calculated on NCCT head was done making dichotomized group using Glasgow Outcome Score (GOS) in death/dependent[
The inclusion criteria were all patients admitted to trauma center, IMS BHU, Varanasi, India, and submitted surgical evacuation of EDH in the Neurosurgery Department between August 1, 2018, and November 1, 2019. Patients who underwent surgery in the first 2 h of reporting to trauma center were included in the study. The exclusion criterion was unknown data concerning the study variables and conservatively managed EDH cases and those who did not give consent for surgery. Two hundred and fifteen patients were included in the study.
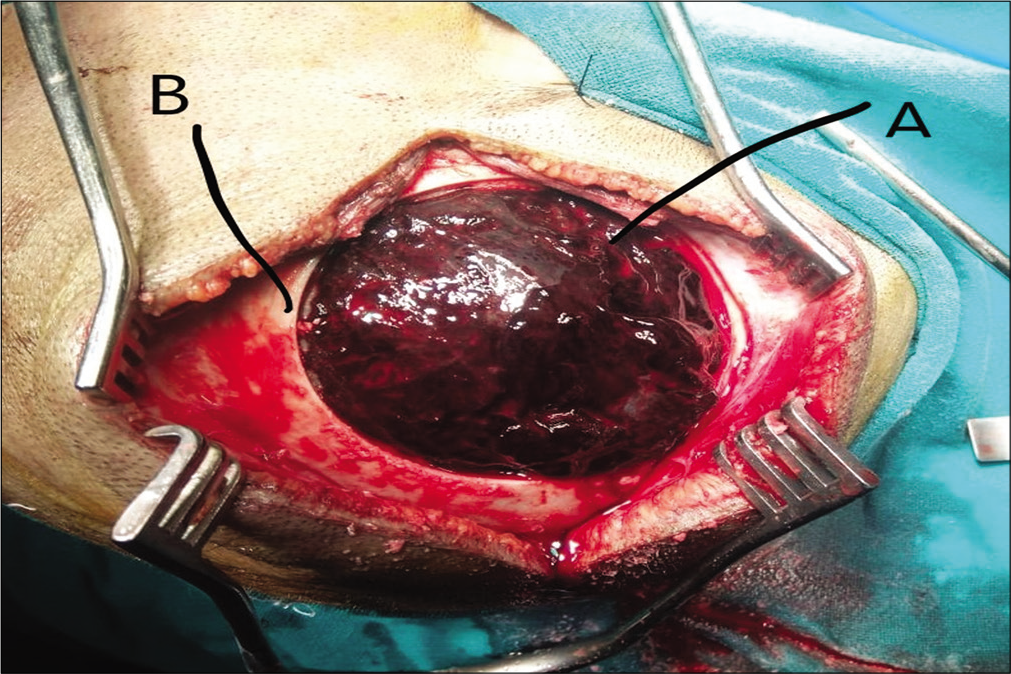
Surgical technique used was craniotomy with EDH evacuation. In cases of temporal EDH or comminuted skull fracture, craniectomy was done. In patients on anticoagulation therapy, fresh frozen plasma was used to correct the INR, and in those under antiplatelet therapy, pools of platelets were used according to surgeon preference. Postoperative ICU care was provided. Informed consent was taken from all operated cases by patient or patient attendants (in cases, in which patient was not able to consent, namely, poor GCS).
Data were collected retrospectively by analyzing medical, surgical, and radiological records, of 253 operated patients submitted in medical record department, IMS BHU, Varanasi, India, between August 1, 2018, and November 1, 2019. Statistical tests were done using GraphPad Prism version 8.3.0 software. P value was taken at 0.05. Null hypothesis of no significant difference in mean and alternative hypothesis of significant difference between means was taken. Test of significance was done using unpaired t-test with Welch’s correction. Outcome analysis of giant EDH was done using Pearson’s Chi-square test and one-way ANOVA test.
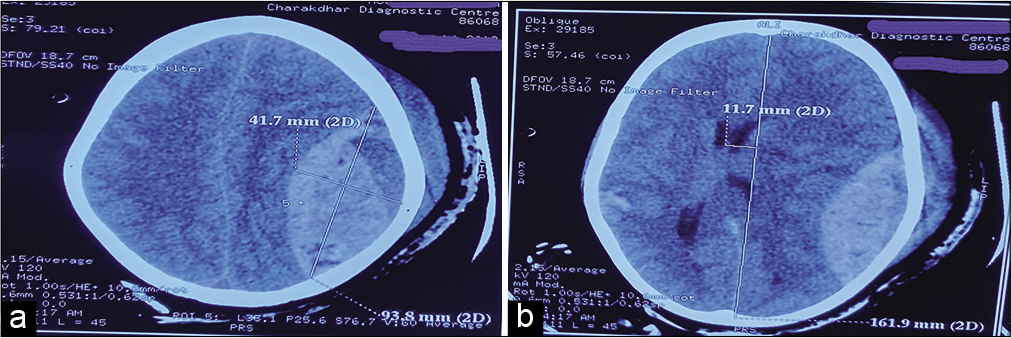
The volume of the EDH (EDHV) was calculated using the Peterson and Espersen equation.[
Volume of EDH = a × b × c × 0.5
Where, a, b, and c represented diameters of the hematoma in the sagittal, axial, and coronal planes.
RESULTS
Dichotomized GOS analysis of GOS 4–5 versus GOS 1–3 with respect to clot volume
Average mean clot volume of GOS 4–5 was 43 ml (n = 230) while that for GOS 1–3 was 79.68 ml (n = 23). There was a significant (P < 0.001) difference in means between two groups. Based on it, we took clot volume ≥80 ml for further analysis of giant EDH [
Analysis of giant EDH (volume ≥ 80 ml)
Age distribution
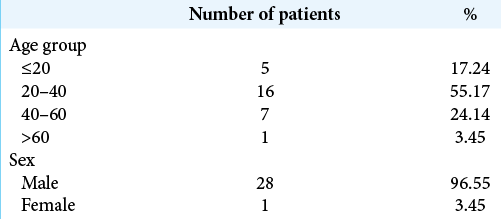
In this study, the most common patients operated for giant EDH were in the age group of 20–40 (n = 16, 55.17%) followed by the age group of 40–60 (n = 7, 24.14) [
Sex distribution
In this study, most of the patients operated for giant EDH were males (n = 28, 96.55%). Male-to-female ratio was 28:1 [
Mode of injury
In this study, most common mode of injury was RTA (n = 23, 79.31%) followed by assault (n = 5, 17.24%) and fall from height (n = 1, 3.45%).
Best motor response (M status)
In this study, most common M status was M2 (n = 9, 31.03 %) followed by M3 (n = 7, 24.14 %) and M4 (n = 7, 24.14), respectively.
Severity of injury
In this study, patients with giant EDH were having moderate, GCS 9–13, (n = 6, 20.69%) and severe, GCS ≤ 8 (n = 23, 79.31 %). No patient was having mild head injury, GCS 14–15.
Clinical signs
In this study, pupillary changes (bilateral) were present in 19 patients (65.52%), pupillary changes (unilateral) were present in 10 patients (34.48%), and bradycardia was present in 7 patients (24.14%).
Site of giant EDH
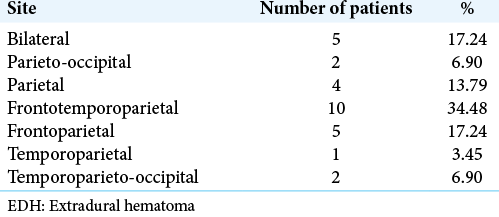
In this study, most common site of giant EDH was frontotemporoparietal (n=10, 34.48%) followed by frontoparietal (n = 5, 17.24%) and parietal (n = 4, 13.79%) [
Side of giant EDH
In this study, the right-sided giant EDH was present in 12 patients (41.38%), left-sided giant EDH in 11 patients (37.93%), and bilateral giant EDH was seen in 6 patients (20.69%).
Source of bleeding in giant EDH
In this study, the source of bleeding was dural venous sinus bleed in 20 patients (68.96%) followed by middle meningeal arterial bleed in 9 patients (31.04%) [
Volume of clot evacuated during surgery
Intraoperative clot evacuated was >140 ml in 10 patients (34.48%), 120–140 ml in 7 patients (24.14%), 100–120 ml in 8 patients (27.59%), and 80–90 ml in 4 patients (13.79%).
CT findings
In all patients, midline shift and herniation were present.
GOS
In this study, GOS of 1 was observed in 10 patients (34.48%), GOS of 2 was seen in 6 patients (20.69%), GOS of 3 was seen in 5 patients (17.24%), GOS of 4 was seen in 7 patients (24.14%), and GOS of 5 was seen in 1 patients (3.45 %).
In this study, we found that GCS at admission (P < 0.0001), pupillary changes (P = 0.0032), and best motor response (P < 0.0001) was significantly (P < 0.05) associated with outcome following surgery for giant EDH. In contrast, age, gender of patients, and severity of injury were not found to be significantly associated with outcome following surgery [
DISCUSSION
EDHs are usually located in vicinity of skull fracture. EDH formation is usually rapid over span of few hours from the time of injury but may run a chronic course, detected only days after injury.[
In patients operated on for giant EDH, age was ranged from 6 years to 75 years. Highest numbers of the victims were in the most active period of life, that is, 20–40 years age group (n = 16, 55.17%) followed by 40–60 years age group (n = 7, 24.14%). There is a paucity of data in literature regarding giant EDH age distribution pattern. However, in reported series comprising all EDHs, peak incidence of EDH being in the second decade and the mean age of patients with EDH is between 20 and 30 years of age.[
In the present study, male-female ratio of 28:1 is reflection of our social culture where most of our females are not exposed to external works. We found male dominance (n = 28, 96.55%) over female counterparts (n = 1, 3.45%) in patients operated for giant EDH. There is a paucity of literature regarding sex dominance in patients having giant EDH. In reported series of all EDH cases, the male dominance is recognized over female.[
In this study, road traffic accidents (RTAs) were the most common cause (n = 23, 79.31%) of injury followed by assault (n = 5, 17.24%), leading to the development of giant EDH. It is comparable with many other published series[
In various reported series, EDH is more frequently located in the temporoparietal and temporal region as compared with other locations.[
In this study, we observed the most common best motor response of M2 in 9 patients (31.03 %) followed by M3 (n = 7, 24.14 %) and M4 (n = 7, 24.14 %). We observed poor outcome in operated patients in proportion to low M status. Best motor response at presentation has been identified as the important factors determining outcome in patients of EDH.[
In this study, most of the head injury cases leading to giant EDH were severe (n = 23, 79.31%) followed by moderate (n = 6, 20.69%) head injury. Volume of giant EDH was not statistically correlated with severity of injury in our study.
Bilateral pupillary changes (n = 19, 65.52%) and unilateral pupillary changes (n = 10, 34.48%) in patients with giant EDH indicate herniation and brainstem compression due to very large size of giant EDH hematoma. Bradycardia was present in 7 patients (24.14%) and was also indicative of brainstem compression.
Most common source of bleeding leading to giant EDH in our study was rupture of dural venous sinuses (n = 20, 68.96%) followed by middle meningeal artery (n = 9, 31.04%). In a recent report on EDH in 102 pediatric patients and 387 adults, arterial bleeding identified as the source of EDH in 36% of adults and in 18% of children with EDH.[
Independent predictors of poor surgical outcome as reported in studies are low GCS, associated intracranial lesion, pupillary abnormalities, and raised ICP.[
The significant clinical factor associated with unfavorable outcome in this study was GCS at admission, best motor response, and pupillary status. Age, gender of patients, and severity of injury were not found to be significantly associated with outcome following surgery for giant EDH. GCS at the time of admission or GCS before the surgery is the single most important predictor of outcome in patients with EDH undergoing surgery.[
In this study, there were 10 mortalities (34.48%) with GOS of 1. These indicate poor outcome and increased mortality in postoperative cases of giant EDH (≥80 ml). There is a paucity of literature in regarding postoperative mortality of giant EDH.
CONCLUSION
From this study, we conclude that giant EDH with volume ≥80 ml causes immediate midline shift leading to herniation and brainstem compression leading to raised percentage of mortality and morbidity. Early evacuation of giant EDH carries better prognosis.
Poor GCS at admission, low best motor response status, and pupillary changes are associated with unfavorable outcome in patients with giant EDH.
Male dominance with male:female ratio of 28:1 and peak incidence in 20–40 years of productive population are some other salient features.
Volume of giant EDH was not statistically correlated with severity of injury in our study.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bricolo A, Pasut L. Extradural hematoma: Towards zero mortality. A prospective study. Neurosurgery. 1984. 14: 8-12
2. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of acute epidural haematomas. Neurosurgery. 2006. 58: 52-7
3. Carlos UP, Joas DB, Carneiro L, Antonio R, Egmond ASS, Joas TSM. Epidural haematoma: Analysis of 30 cases. Internet J Emerg Med. 2005. 2: 44-7
4. Chowdhury NK, Raihan MZ, Chowdhury FH, Ashadullah AT, Sarkar MH, Hossain SS. Surgical management of traumatic extradural haematoma: Experiences with 610 patients and prospective analysis. Indian J Neurotrauma. 2008. 5: 75-9
5. Dubey A, Pillai SV, Kolluri SV. Does volume of extradural hematoma influence management strategy and outcome?. Neurology India. 2004. 52: 443-5
6. Ersahin Y, Mutluer S, Guzelbag E. Extradural Haematoma analysis of 146 cases. Childs Nerv Syst. 1993. 9: 96-9
7. Faheem M, Jaiswal M, Ojha BK, Chandra A, Singh SK, Srivastava C. Traumatic pediatric extradural hematoma: An institutional study of 228 patients in tertiary care center. Pediatr Neurosurg. 2019. 54: 237-44
8. Haselsberger K, Pucher R, Auer L. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir (Wien). 1988. 90: 111-6
9. Jones N, Molloy C, Kloeden C, North J, Simpson D. Extradural haematoma: Trends in outcome over 35 years. Br J Neurosurg. 1993. 7: 465-71
10. Joom AJ. The difference in the outcome of surgery for traumatic extradural haematoma between patients who are admitted directly to the neurosurgical unit and those referred from another hospital. Neurosurg Rev. 1997. 20: 227-30
11. Lee EJ, Hung YC, Wang LC, Chung KC, Chen HH. Factors influencing the functional outcome of patients with acute epidural haematomas: Analysis of 200 patients undergoing surgery. J Trauma. 1998. 45: 946-52
12. McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M. Consensus statement on concussion in sport: The 3rd international conference on concussion in sport held in Zurich 2008. Br J Sports Med. 2009. 43: i76-90
13. Mohanty A, Kolluri VR, Subbakrishna DK, Satish S, Chandramouli BA, Das BS. Prognosis of extradural haematomas in children. Pediatr Neurosurg. 1995. 23: 57-63
14. Petersen OF, Espersen JO. Extradural hematomas: Measurement of size by volume summation on CT scanning. Neuroradiology. 1984. 26: 363-7
15. Rengachary SS, Ellenbogen RG.editors. Principles in Neurosurgery. India: Elsevier, Mosby; 2004. p. 2081
16. Uzkan U, Kemaloglu S, Ozates M, Guzel A, Tath M. Analyzing extradural haematomas: A retrospective clinical investigation. Dicle Med J. 2007. 34: 14-9