- Department of Neurosurgery, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Correspondence Address:
Dr. G. Lakshmi Prasad, Room 12, OPD Block, Department of Neurosurgery, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal 576104, Karnataka, India.
DOI:10.25259/SNI_411_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Vyjayanth Reddy, Aseem Pradhan, G. Lakshmi Prasad, Girish Menon. Clinical outcomes and prognostic factors of traumatic basal ganglia hematomas: A 4-year single-center study. 21-Jul-2023;14:251
How to cite this URL: Vyjayanth Reddy, Aseem Pradhan, G. Lakshmi Prasad, Girish Menon. Clinical outcomes and prognostic factors of traumatic basal ganglia hematomas: A 4-year single-center study. 21-Jul-2023;14:251. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12454
Abstract
Background: Traumatic basal ganglia hematomas (TBGH) are rare entities. They are situated in the deep cerebral parenchyma and have also been termed as intermediate coup contusions. Available literature is sparse with regards to the characteristics and prognosis of TBGH. We aim to share our experience in the management, outcomes, and prognostic factors of TBGH.
Methods: A 4-year retrospective study which included all cases of TBGH, except dot contusions (
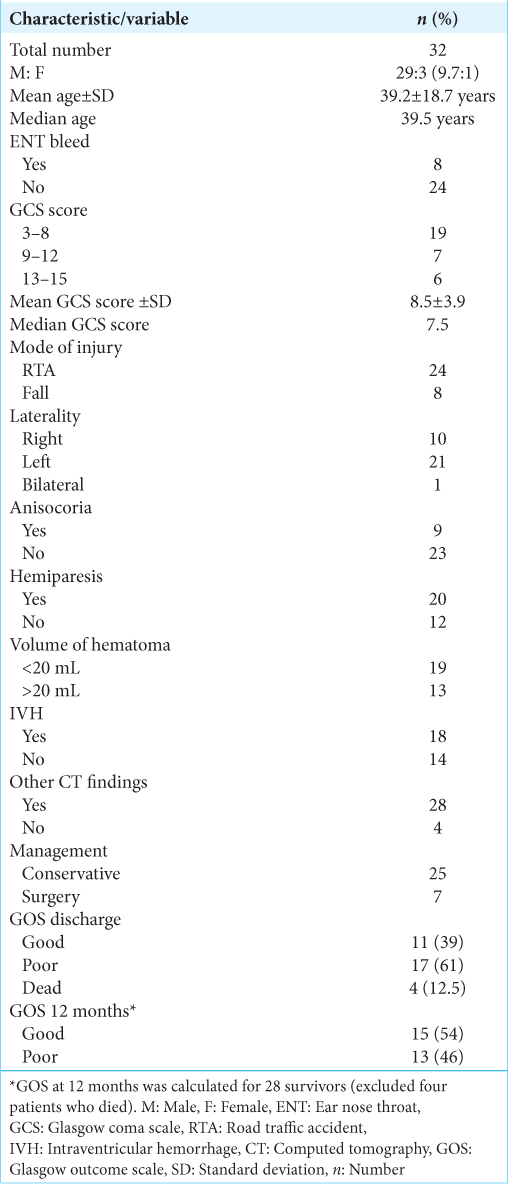
Results: Thirty-two patients were analyzed. The mean age was 39.2 years. Two-thirds were due to road traffic accidents. Around 60% were severe head injuries. The mean Glasgow coma scale (GCS) score at presentation was 8.5. Twenty patients had moderate-to-severe hemiparesis. The mean hematoma volume was 18.1 mL. Associated traumatic intracranial lesions were seen in 28 cases. Only 7 patients (22%) underwent surgery. The mean follow-up was 17.4 months (range 14–34 months). The mortality rate was 12.5% (n = 4). Among the survivors, only 39% (n = 11) had good outcomes at discharge which showed modest improvement to 54% (n = 15) at 12 months.
Conclusion: Our study noted that poor admission GCS scores, poor motor response, presence of significant hemiparesis, and larger hematoma volumes (>20 mL) correlated with poor outcomes at 12 months. The overall outcomes have been mostly unfavorable as observed in majority of studies due to deeper location of these hematomas, high proportion of severe head injuries, and high proportion of residual weakness in survivors.
Keywords: Basal ganglia hematomas, Glasgow outcome scale, Intermediate contusions, Outcome and prognosis, Traumatic
INTRODUCTION
In the subset of trauma patients, traumatic brain injury (TBI) is the leading cause of death and disability and the majority of this burden is observed in low- and middle-income countries.[
MATERIALS AND METHODS
This was a single-center 4-year retrospective study conducted between January 2015 and December 2018 at Kasturba Medical College, Manipal, India.
Inclusion criteria
Patients with basal ganglia region hemorrhage noted on computed tomography scan and an unequivocal history of trauma (RTA, fall from height, and assault) were included in the study.
Exclusion criteria
The following criteria were excluded from the study:
Patients with spontaneous basal ganglia hematomas, such as due to hypertension or coagulation abnormalities Absent brainstem reflexes on arrival Dot-like contusions with volume <2 mL.
Patients’ data were retrieved through case sheets, operative records, discharge summaries, outpatient clinic notes, and telephonic conversations. The following variables were noted for analysis: age, sex, admission Glasgow Coma Scale (GCS) score (mild: 13–15, moderate: 9–12, and severe: <8), mechanism of injury, radiological findings (EDH/SDH/DAI/Intraventricular haemorrhage [IVH]), type of management, follow-up, and outcomes. Patients of severe head injury, with signs of raised intracranial pressure (ICP), and not responding to initial conservative measures underwent surgical evacuation. All patients were regularly followed up on an outpatient basis, at 1 month, 3–6 months, and 12 months after discharge. Outcomes were assessed using the Glasgow Outcome Scale (GOS) score at discharge and follow-up. We classified the GOS scores into good (good recovery and moderate disability) and poor outcomes (severe disability, vegetative state, or death).
Statistical analysis
Statistical analyses were carried out using SPSS software version 21.0. Qualitative variables were presented as frequency and percentages. Quantitative variables were presented as mean (standard deviation) or median (range) depending on the distribution of data. All admission variables were correlated with GOS score at discharge and at 12-month follow-up. Age and hematoma volumes were dichotomized based on the mean values. Chi-square test was used to perform the statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
After exclusion, 32 patients were analyzed. The mean and median ages were 39.2 years (standard deviation [SD] ± 18.7) and 39 years (range 7–79 years), respectively. Half of them were below 40 years. The male: female ratio was 9.6:1 (29:3). Two-thirds were due to RTA (n = 24). There were 19 severe, seve moderate, and six mild head injuries. The mean GCS at presentation was 8.5 (±3.5 SD) and the median GCS was 7.5. Eight patients had presented with nasal/ear bleed and three patients had seizures at presentation. Asymmetrical and nonreactive pupils were noted in 9 (28%) cases. Twenty patients (62%) had moderate-to-severe hemiparesis.
The mean hematoma volume was 18.1 mL and the median volume was 8.6 mL (range, 2.5–70 mL). There were 21 (65%) left-sided, 9 (28%) right-sided, and two bilateral hematomas. IVH was present in 20 cases (62%). Associated traumatic intracranial lesions (EDH, SDH, contusions, DAI, and subarachnoid hemorrhage [SAH]) were seen in 28 cases (87%). Most of the patients (n = 25) (78%) were managed conservatively and only 7 patients (22%) underwent surgery.
Follow-up and outcomes
The mean follow-up was 17.4 months (range 14–34 months) and all patients had a minimum of 12-month FU. The mortality rate was 12.5% (n = 4) and all were severe head injuries. Among the survivors, 61% (n = 17) patients had poor outcomes and 39% had good outcomes at discharge. At 1-year follow-up, there was a modest increase in the proportion of good outcomes (GOS 4 and 5), from 39% (n = 11) to 54% (n = 15). Of the 20 patients with hemiparesis, 12 had residual weakness at 1-year follow-up.
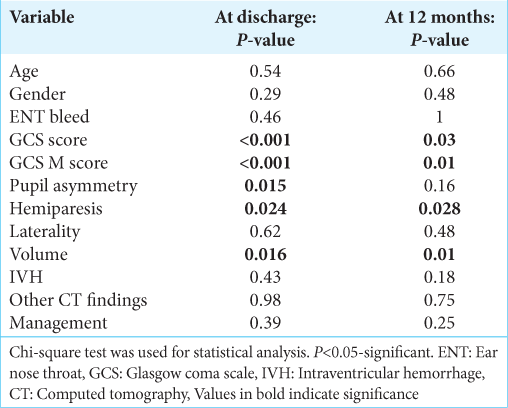
We tried to correlate various variables with patient outcomes, at discharge and 12 months. At discharge, the following variables had statistically significant association with poor outcomes, namely, poor admission GCS score (P < 0.001), poor motor response component of GCS (P < 0.001), pupil nonreactivity (P = 0.015), presence of hemiparesis (P = 0.024), and hematoma volume >20 mL (P = 0.016). At 12 months, except pupillary asymmetry, all the other variables were significantly associated with poor outcome namely, admission GCS score (P = 0.003), GCS motor component (P = 0.01), hematoma volume (P = 0.01), and paresis (P = 0.028). The remaining variables such as age, gender, presence of ENT bleed, laterality, associated CT findings, presence of IVH, and mode of management did not show statistical association with outcomes.
DISCUSSION
In clinical studies, the incidence of TBGH has been observed to be around 2–3% of closed head-injured patients; however, autopsy series indicate a higher incidence, ranging between 10% and 12%.[
Pathophysiology of injury
Multiple theories have been proposed to elucidate the pathophysiology of such lesions. Mosberg and Linberg observed that shearing forces are produced when sufficient impact directed toward the tentorium is delivered to the vertex/forehead/occipital area when the head is in motion, thus causing stretching and tearing of pallidal branches of the anterior choroidal artery and striatal branches of MCA which in turn produce hemorrhage in the basal ganglia region.[
Clinicoradiological features
They are predominantly seen in young adults (3rd and 4th decades) and uncommonly reported in pediatric age group.[
Boto et al. retrospectively analyzed 37 cases of severe TBI with TBGH. About 94% were due to RTA. Skull fracture was present in 10 (43%) of the 23 patients in whom skull X-rays were obtained. Coagulation disorders were present in 32 (86%) cases. CT findings included DAI in 27 patients (73%), IVH in 22 patients (59%), and SAH in 16 patients (43%). Four patients (11%) underwent surgical evacuation.[
Takeuchi retrospectively analyzed 20 cases of TBGH. About 60% were due to RTA (n = 12) and rest due to FFH (n = 8). The mean GCS score was 7.5. Skull fractures were seen in 5 (25%) cases. They noted a high frequency of putaminal involvement (n = 15). The mean hematoma volume was 10.7 mL. Associated lesions were as follows: SAH (n = 10), focal contusions (n = 9), SDH (n = 5), IVH (n = 4), and DAI (n = 5). Six patients (30%) underwent surgery and rest were conservatively managed.[
Kurwale et al. analyzed 21 pediatric cases of TBGH. High velocity RTA (52%) and falls (38%) were the injury mechanisms. The mean GCS score was 6 and 4.8 (overall and in severe HI subgroup). Majority (76%) were severe HI. Ten (47%) had associated injuries and skull fractures were noted in seven cases. Only 3 (14%) cases underwent surgical evacuation.[
With regards to clinicoradiological features, our study observations are similar to most of the previously published studies.
Management and outcomes
Treatment options for patients with TBGH include conservative management, craniotomy, and stereotactic/ ultrasound-guided aspiration. In all the previous studies, majority of the cases were managed conservatively. In general, the authors have recommended surgical evacuation for hematoma volume ˃ 25 mL.[
Although there have been mixed observations regarding the outcomes of TBGH, majority of the authors have noted poor outcomes in their patients.[
Similar results (high rates of poor outcomes and mortality rates) were reported by other authors as well.[
However, one study by Kumar et al. reported good outcomes (GOS 4 or 5) in all their patients with zero mortality. This was because of the favorable admission characteristics in their study, namely, mean GCS score of 10 and mean volume of 13.2 mL.[
In our study, the mortality was rather low as compared to other studies. We feel that one of the main reasons for this is that we had a lesser proportion of severe head injuries in comparison with other studies. In the study by Boto et al., all 100% were severe TBI while it was 72% as noted by Kurwale et al. In terms of GCS scores, the mean GCS score was 7.5 and 6, respectively, in the studies by Takeuchi et al. and Kurwale et al., while it was 8.5 in our study. Furthermore, more than half of the patients in the study by McPherson et al. had features of raised ICP (effacement of basal cisterns and >5 mm midline shift) and Kurwale et al. reported that five patients died primarily due to raised ICP. These would have also contributed to the high mortality in these studies. The exact mortality rate has not been mentioned by McPherson et al., however, they observed that two-thirds were either dead or severely disabled at 6 months. Apart from the mortality rates, the proportion of poor outcomes (severely disabled and vegetative state) in our study was similar to the findings of other studies.[
With regards to prognostic factors, poor admission GCS scores, pupillary asymmetry, presence of midline shift, larger hematoma volume, enlarging hematomas, and hematomas with raised ICP were the common factors associated with poor outcomes in all of the previous studies.[
Merits and limitations
Merits
We analyzed 32 patients with strict inclusion and exclusion criteria (excluding dot contusions) and all patients were followed for a minimum of 12 months which is not commonly reported in other studies.
Limitations
The limitations of the study are retrospective nature of the study, single-center study, and the relatively smaller number of subjects for statistical analysis.
CONCLUSION
TBGHs are rare lesions encountered in clinical practice. Majority are due to high velocity trauma and most of them have severe head injuries. They are mostly managed conservatively with surgery reserved for larger hematoma volumes. Our study noted poor admission GCS scores, poor motor response component of GCS score, presence of notable hemiparesis, and larger hematoma volumes (>20 mL) to correlate with poor outcomes at 12 months. The overall outcomes have been mostly unfavorable as observed in majority of studies due to deeper location of these hematomas, high proportion of severe head injuries, and high proportion of residual weakness in survivors.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adams G, Doyle D, Graham DI, Lawrence AE, McClellan DR. Deep intracerebral (basal ganglion) hematomas in fatal non-missile injury in man. J Neurol Neurosurg Psychiatry. 1986. 49: 1039-43
2. Bhargava P, Grewal SS, Gupta B, Jain V, Sobti H. Traumatic bilateral basal ganglia hematoma: A report of two cases. Asian J Neurosurg. 2012. 7: 147-50
3. Boto GR, Lobato RD, Rivas JJ, Gomez PA, de la Lama A, Lagares A. Basal ganglia hematomas in severely head injured patients: Clinicoradiological analysis of 37 cases. J Neurosurg. 2001. 94: 224-32
4. Fujioka M, Maeda Y, Okuchi K, Taoka TK. Secondary change in the substantia nigra induced by incomplete infarct and minor hemorrhage in the basal ganglia due to traumatic middle cerebral arterial dissection. Stroke. 1999. 30: 1975-7
5. Gupta N, Kankane VK, Gupta TK. Outcome of Traumatic bilateral basal ganglia Hemorrhage: Rarest entity: Prospective study of five cases: Single institutional Experience. Roman Neurosurg. 2018. 32: 322-31
6. Jang KJ, Jwa CS, Kim KH, Kang JK. Bilateral traumatic hemorrhage of the basal ganglia. J Korean Neurosurg Soc. 2007. 41: 272-4
7. Jayakumar PN, Kolluri VR, Basavakumar DG, Arya BY, Das BS. Prognosis in traumatic basal ganglia hematoma. Acta Neurochir. 1989. 97: 114-6
8. Jellinger K. Traumatic basal ganglia hemorrhage. Neurology. 1940. 40: 862-3
9. Katz DI, Alexander MP, Seliger GM, Bellas DN. Traumatic basal ganglia hemorrhage: Clinicopathologic features and outcome. Neurology. 1989. 39: 897-904
10. Kumar S, Jha D, Abbey P, Mishra V, Handa A. Outcome of traumatic basal ganglia hemorrhage. Internet J Neurosurg. 2008. 6: 1-5
11. Kurwale NS, Gupta DK, Mahapatra AK. Outcome of pediatric patients with traumatic basal ganglia hematoma: Analysis of 21 cases. Pediatr Neurosurg. 2010. 46: 267-71
12. Lee JP, Wang AD. Post-traumatic basal ganglia hemorrhage: Analysis of 52 patients with emphasis on the final outcome. J Trauma. 1991. 31: 376-80
13. Maki Y, Akimoto H, Enomoto T. Injuries of basal ganglia following head trauma in children. Childs Brain. 1980. 7: 113-23
14. Massenburg BB, Veetil DK, Raykar NP, Agrawal A, Roy N, Gerdin M. A systematic review of quantitative research on traumatic brain injury in India. Neurol India. 2017. 65: 305-14
15. McPherson P, Teasdale E, Dharkar S, Allerdyce E, Galbraith S. The significance of traumatic hematoma in the region of basal ganglia. J Neurol Neurosurg Psychiatry. 1986. 49: 29-34
16. Mosberg WH, Lindenberg R. Traumatic hemorrhage from the anterior choroidal artery. J Neurosurg. 1959. 16: 209-21
17. Munemoto S, Komai T, Aizumi S. Traumatic hemorrhage in the basal ganglia in the child: Five cases. No Shinkei Geka. 1985. 13: 1027-33
18. Rubiano AM, Carney N, Chesnut R, Puyana JC. Global neurotrauma research challenges and opportunities. Nature. 2015. 527: S193-7
19. Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Yatsushige H, Shigeta K. Traumatic basal ganglia hematomas: An analysis of 20 cases. Acta Neurochir Suppl. 2013. 118: 139-42
20. Yanaka K, Egashira T, Maki Y, Takano S, Okazaki M, Matsumaru Y. Bilateral traumatic haemorrhage in the basal ganglia: report of two cases. No Shinkei Geka. 1991. 19: 369-73
21. Zimmerman RA, Bilaniuk LT, Genneralli T. Computed tomography of shearing injuries of the cerebral white matter. Radiology. 1978. 127: 393-6