- Department of Critical Care Medicine, Nara Prefecture General Medical Center, Nara, Japan.
- Department of Neurosurgery, Nara Prefecture General Medical Center, Nara, Japan.
Correspondence Address:
Kiyoshi Takemoto, Department of Critical Care Medicine, Nara Prefecture General Medical Center, Nara, Japan.
DOI:10.25259/SNI_903_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kiyoshi Takemoto1, Tomonori Yamamoto1, Hiroyuki Hashimoto2, Takeshi Matsuyama1, Kazuaki Atagi1. Clinical significance of cerebrospinal fluid presepsin as adjunctive biomarker for postneurosurgical meningitis: A single-center prospective observational study. 26-Jan-2024;15:26
How to cite this URL: Kiyoshi Takemoto1, Tomonori Yamamoto1, Hiroyuki Hashimoto2, Takeshi Matsuyama1, Kazuaki Atagi1. Clinical significance of cerebrospinal fluid presepsin as adjunctive biomarker for postneurosurgical meningitis: A single-center prospective observational study. 26-Jan-2024;15:26. Available from: https://surgicalneurologyint.com/surgicalint-articles/12722/
Abstract
Background: Postneurosurgical meningitis (PNM) is a serious complication in neurocritical care patients, leading to clinical deterioration and worsening outcomes. Accurate diagnosis of PNM is often difficult due to the lack of definitive diagnostic criteria. This study investigates the potential utility of cerebrospinal fluid (CSF) presepsin (PSP), blood PSP, and the CSF/blood PSP ratio as adjunctive biomarkers for the diagnosis of PNM.
Methods: We conducted a single-center prospective observational study at Nara Prefecture General Medical Center in Nara, Japan, from April 2020 to March 2022. The postoperative neurosurgical patients with suspected PNM were included in the study and divided into PNM and non-PNM groups. We evaluated the sensitivity, specificity, area under curves (AUCs), positive predictive value (PPV), and negative predictive value (NPV) for the diagnosis of PNM with CSF PSP, blood PSP, and CSF/blood PSP ratio compared in the two groups.
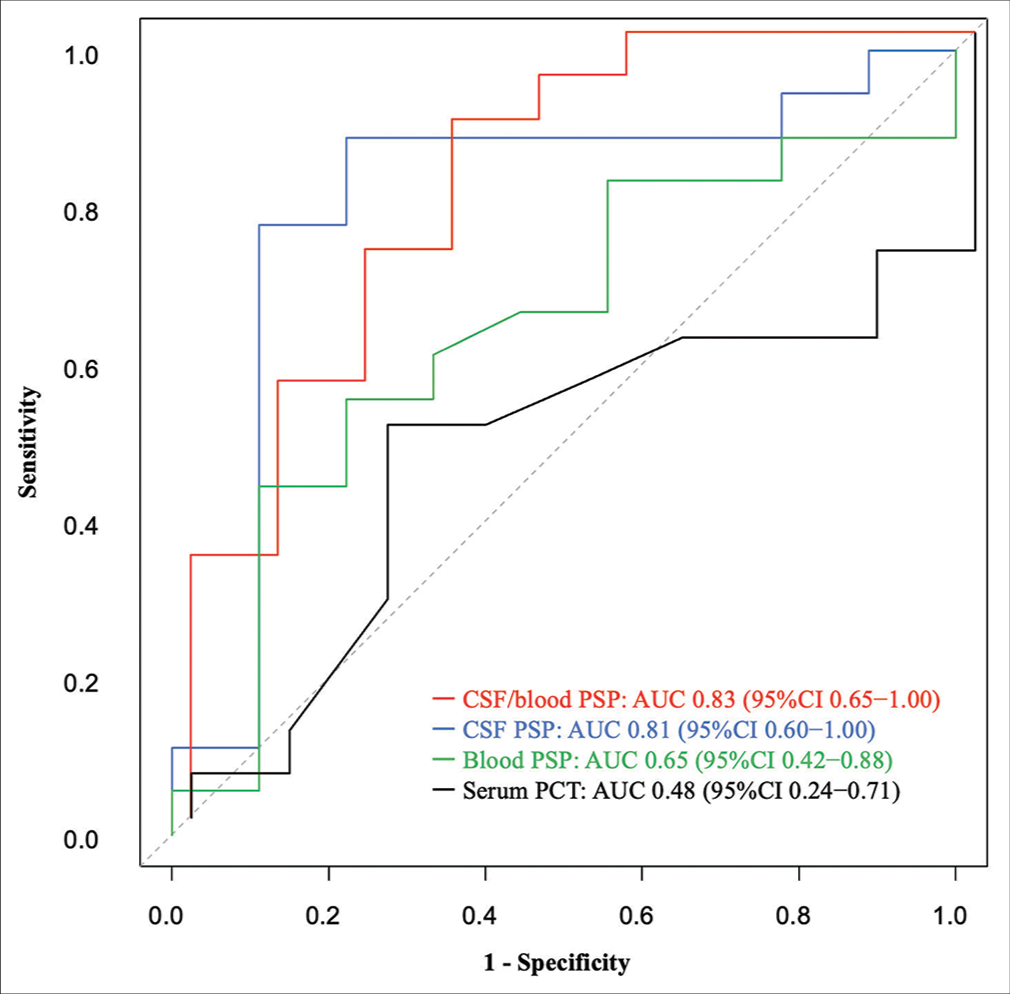
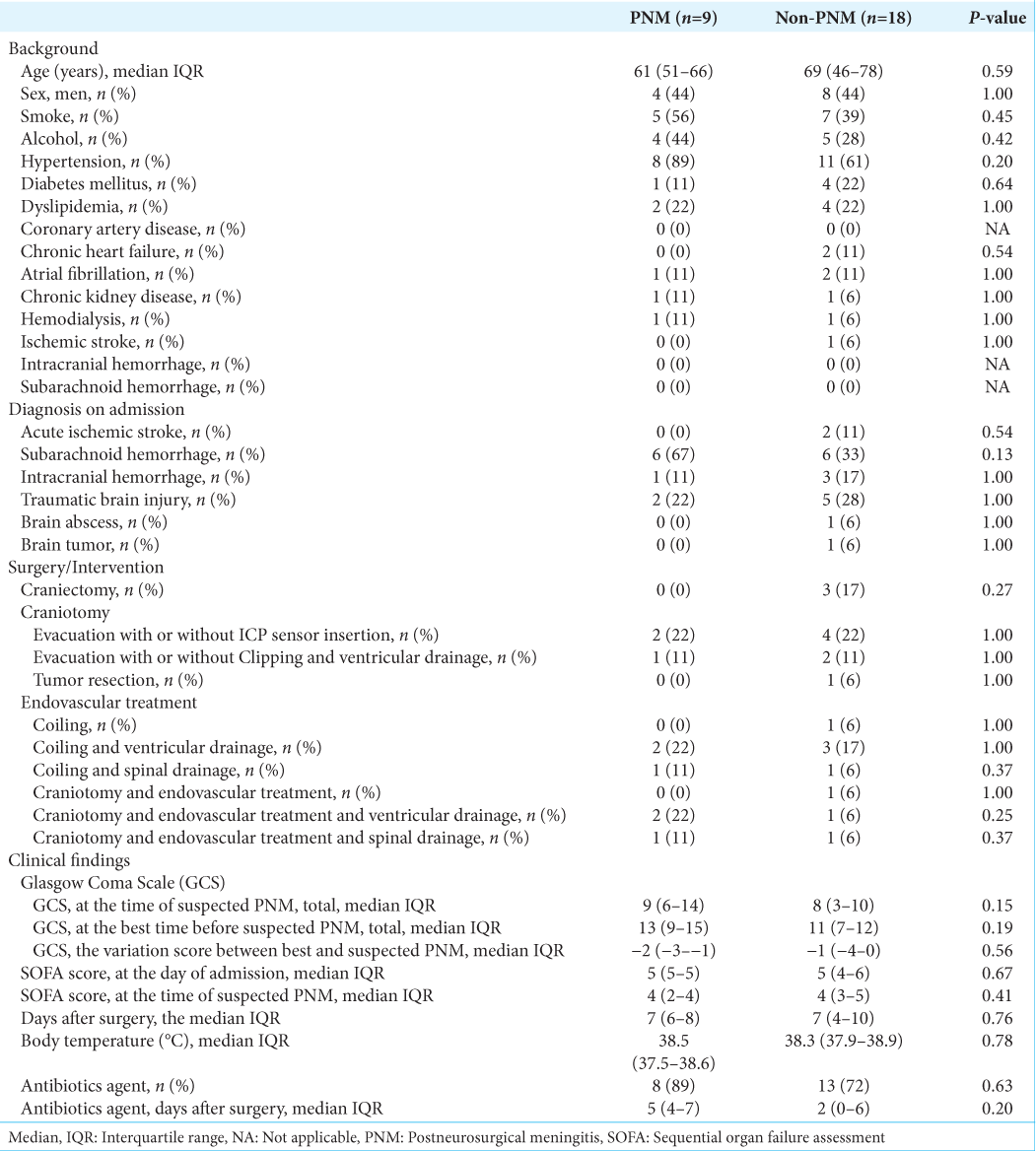
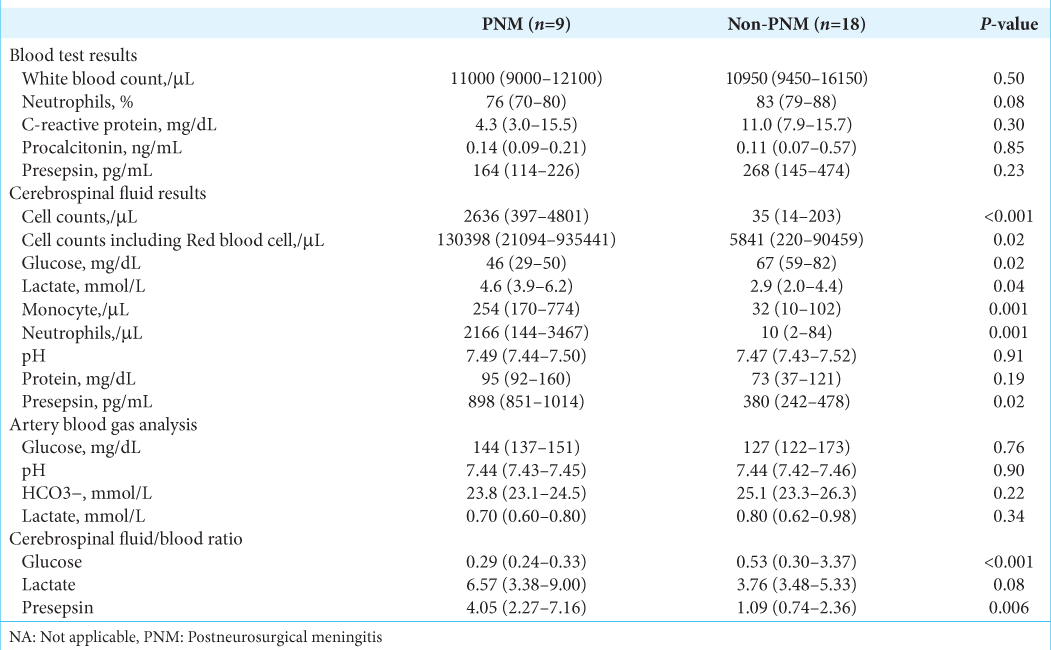
Results: We screened 241 consecutive patients with postoperative neurosurgery. Diagnosis of PNM was suspected in 27 patients, and the clinical diagnosis was confirmed in nine patients. The results of CSF PSP (cutoff: 736 pg/mL) for the diagnosis of PNM were sensitivity 89%, specificity 78%, PPV 67%, NPV 93%, AUC 0.81 (95% confidence interval [CI], 0.60–1.00), blood PSP (cut-off: 264 pg/mL) was 56%, 78%, 56%, and 78%, 0.65 (95% CI, 0.42–0.88), and those of CSF/blood PSP ratio (cutoff: 3.45) was 89%, 67%, 57%, and 92%, 0.83 (95% CI, 0.65–1.00).
Conclusion: Elevated CSF PSP and CSF/blood PSP ratio may be associated with PNM and could serve as valuable adjunctive biomarkers for improving diagnostic accuracy.
Keywords: Biomarker, Cerebrospinal fluid, Diagnosis, Postneurosurgical meningitis, Presepsin
INTRODUCTION
Postneurosurgical meningitis (PNM) is one of the most serious complications associated with high mortality rates, severe neurological sequelae, prolongation of hospital stay, and costs.[
On the other hand, presepsin (PSP: soluble cluster of differentiation 14 [CD14] subtype) is one of the fragments of CD14 that is produced by intracellular cathepsin D and other factors when bacteria are phagocytosed by monocytes/macrophages after bacterial infection.[
MATERIALS AND METHODS
Ethical approval of the study protocol
This study was conducted following the principles of the Declaration of Helsinki. The study was approved by the Institutional Review Board of Nara Prefecture General Medical Center, Nara, Japan (registration number: 517). Written informed consent was obtained from all patients on their initial admission to the hospital or before surgery.
Study population
This single-center prospective observational study was conducted between April 01, 2020, and March 31, 2022, in the intensive care unit at Nara Prefecture General Medical Center in Japan. Patients were enrolled after neurosurgical treatment, including craniectomy, craniotomy, irrigation, internal or external ventricular and lumbar catheter insertion, and endovascular. The patients suspected of PNM were analyzed in this study. We retrospectively divided these patients into two groups (PNM group and non-PNM group) based on the diagnosis criteria of PNM mentioned below. The patients suspected of PNM were to be evaluated for both bacterial and aseptic meningitis.
Inclusion and exclusion criteria
We enrolled all patients of age 18 years or older who had undergone neurosurgery. Among the patients, those with fever (body temperature >38.3°C) and/or unexplained neurologic deterioration were included in this study as suspected PNM. Patients were excluded under the age of 18 years and postcardiac arrest syndrome. CSF and blood samples, including culture and PSP and serum procalcitonin (PCT), were collected at the same time when PNM was suspected. When the CSF culture was positive, the definitive diagnosis of PNM was confirmed regardless of the value of other CSF findings. When the CSF culture was negative, PNM was diagnosed based on clinical diagnosis. The criteria for clinical diagnosis of PNM in this study were defined as prolonged or new onset altered consciousness, reduction in Glasgow Coma Scale,[
Data sampling
Demographic data, clinical parameters, and laboratory findings were collected from medical records, including age, sex, medical history, laboratory results, CSF findings, surgical/intervention details, antibiotics, duration of antibiotic treatment, postoperative days, and Sequential Organ Failure Assessment scores. Clinical data were reviewed independently by two study physicians who were blinded to biomarker measurements, including CSF PSP, blood PSP, CSF/blood PSP ratio, and serum PCT. PSP levels were measured using a fully automatic immunoassay analyzer (PATHFAST, LSI Medience, Tokyo, Japan) according to the manufacturer’s instructions, and the lower limit of detection was set to 20 pg/mL.
Outcome measures
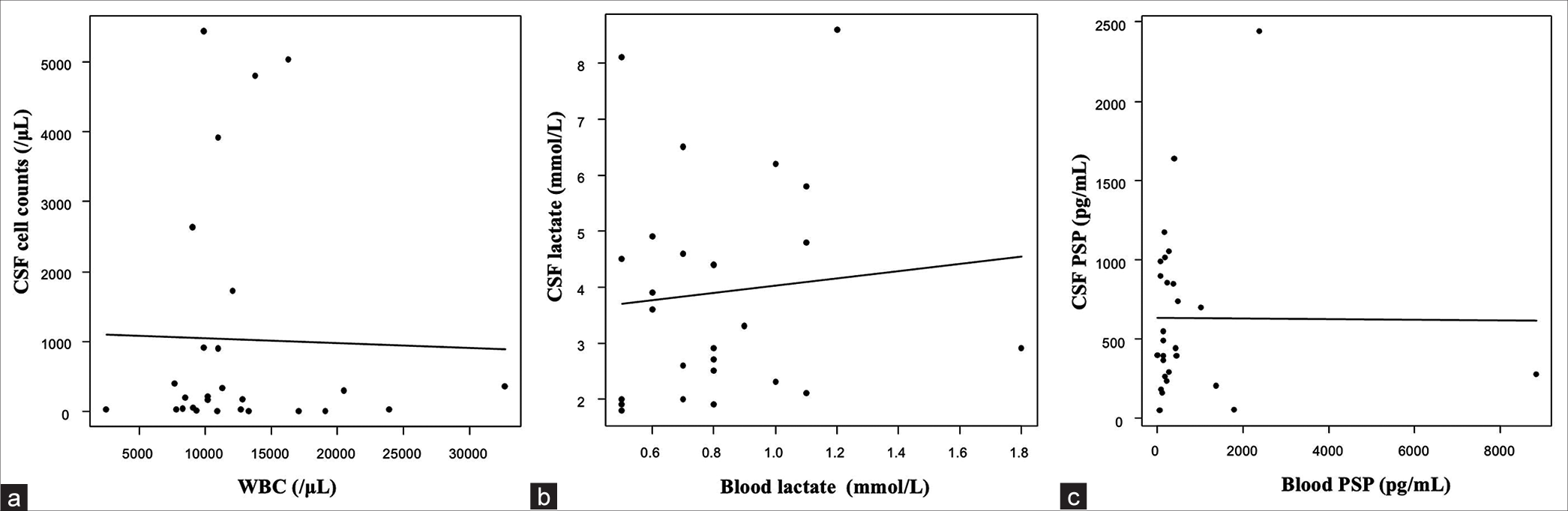
Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic performance of PSP in CSF and blood, CSF/blood PSP ratio, and serum PCT. Cutoff values were determined for each biomarker using the Youden index (corresponding to the maximum of the sum “sensibility + specificity”). Based on cutoff values, prognostic parameters (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) were also calculated. Moreover, linear regression analyses were used to evaluate the correlation between blood contamination of CSF after neurosurgery.
Statistical analysis
All baseline characteristics, clinical variables, and laboratory parameters were compared between patients diagnosed with PNM and non-PNM. The distribution of each variable was compared between the two groups. Continuous variables (expressed as median [interquartile range]) were compared with the Student’s t-test or the Wilcoxon test as appropriate. Categorical variables (expressed as the number [%]) were compared with χ2 or Fisher’s exact tests. All statistical analyses were performed with the EZR software program, version 1.41 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics. A two-sided P < 0.05 was considered statistically significant.
RESULTS
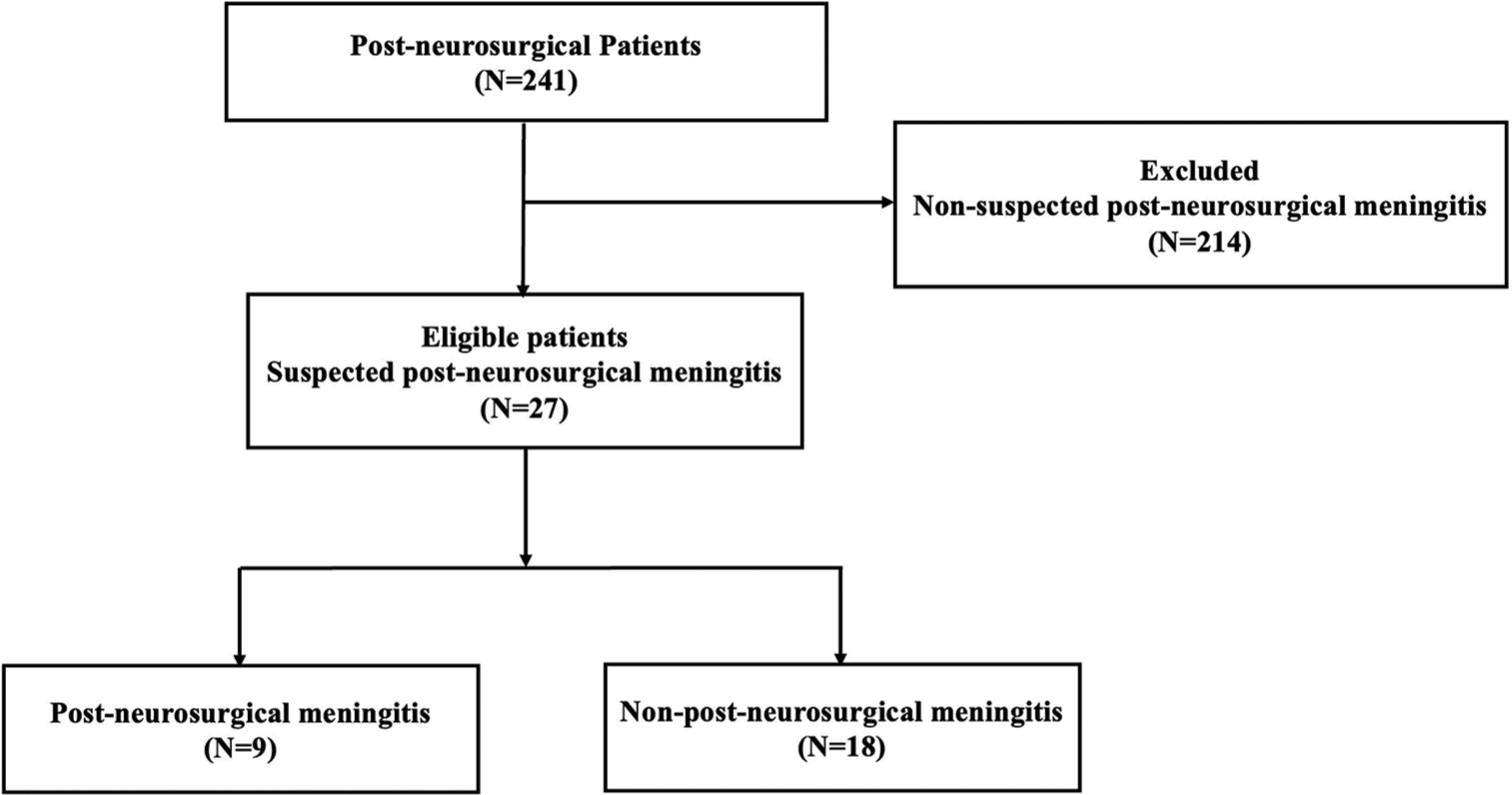
This study screened 241 consecutive patients after neurosurgery. A total of 27 patients with suspected PMN were included in this study [
Figure 2:
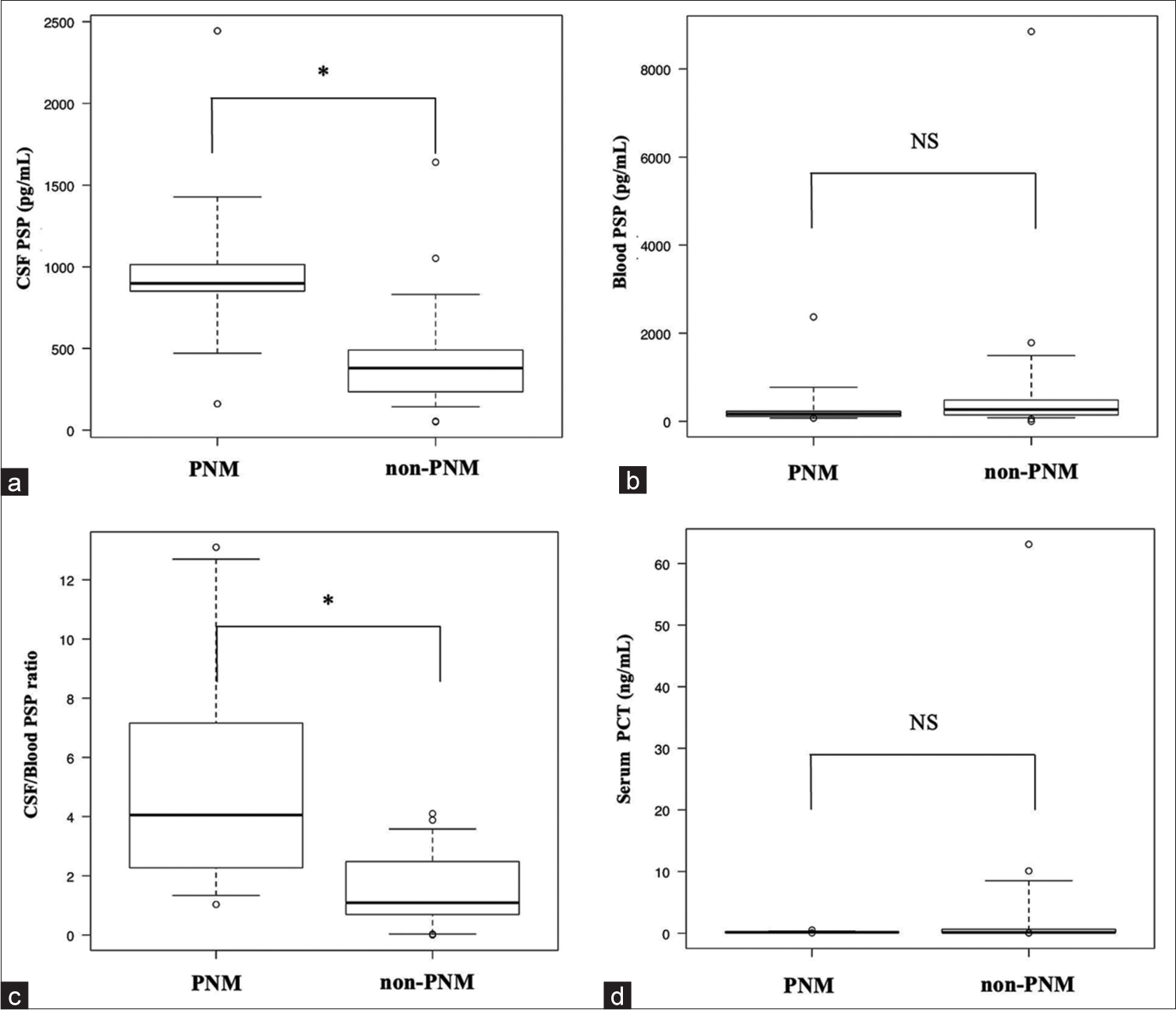
Median values of diagnostic markers of post-neurosurgical meningitis in each group. (a) CSF PSP. (b) Blood PSP. (c) CSF/blood PSP ratio. (d) Serum PCT. Lines denote median values; boxes represent 25th–75th percentiles, and whiskers indicate the range. *P < 0.05, NS: Not significant, CSF: Cerebrospinal fluid, PSP: Presepsin, PCT: Procalcitonin, PNM: Postneurosurgical meningitis.
DISCUSSION
Reliable adjunctive biomarkers with high sensitivity and specificity are needed to assist with the diagnosis of PNM. CSF PSP was reported as one of the useful adjunctive biomarkers for bacterial meningitis mentioned before; however, there was no report to evaluate the CSF/blood PSP ratio for the diagnosis of PNM. This is the first prospective study to evaluate the utility of the CSF/blood PSP ratio in patients with PNM. There were no significant differences in blood PSP between the PNM and non-PNM groups. However, the CSF PSP and CSF/blood PSP ratio at the time of PNM suspicion in the PNM group were significantly higher than in the non-PNM group. Furthermore, ROC analyses revealed that the AUCs of CSF PSP and CSF/blood PSP ratio were higher than blood PSP or serum PCT. In the previous study, the normal range for CSF PSP was 50– 100 pg/mL.[
It should consider the possibility of blood entering the CSF due to rupture of the vascular cavity or blood-brain barrier (BBB) in injuries related to the primary disease, such as traumatic brain injury, subarachnoid hemorrhage, intracranial hemorrhage, or neurosurgical procedures. A combination of disrupted BBB and systemic infection can be a cause of elevated levels of CSF PSP. Severe systemic infection and sepsis can also increase the level of CSF PSP in the absence of bacterial meningitis. That is because PSP synthesized in the blood can cross disrupted BBB, and mediators of systemic inflammation can stimulate glial cells for CSF PSP secretion.[
Figure 4:
Correlation between blood and cerebrospinal fluid (CSF) using linear regression analyses. (a) Blood white blood cell (WBC) and CSF cell counts (r = −0.007, P = 0.90), (b) Blood lactate and CSF lactate (r = 0.65, P = 0.63), (c) Blood PSP and CSF PSP (r = 0.002, P = 0.97). CSF: Cerebrospinal fluid, WBC: White blood cell, PSP: Presepsin.
This study has several limitations. First, there were no patients who presented a positive CSF culture, and there was the absence of microbiological evidence to support the definitive diagnosis of PNM in this study. Second, perioperative antibiotic prophylaxis was used in all patients, which might mask infection and influence the negative CSF culture results. Third, it was conducted in a single center, introducing a potential selection bias such as facility and region. Moreover, uncontrolled confounding factors may exist. Fourth, medication changes and procedures may have contributed to the outcomes in the postintervention. Fifth, we cannot exclude the possibility that changes in the patient population impacted their outcomes. For example, comorbidities were not examined in the present study. Finally, the sample size in this study was very small. Due to the low incidence of PNM in post-neurological intervention, further prospective studies in a large study population with multicenter settings are necessary to clarify the relationship between CSF PSP, blood PSP, and CSF/blood PSP in patients with PNM.
CONCLUSION
The CSF PSP and CSF/blood PSP ratio had a moderate to high AUC and sensitivity for the clinical diagnosis of PNM, suggesting that it might be an adjunctive biomarker for clinical diagnosis. Combining these markers with general conditions and CSF findings of previous criteria for PNM may lead to more appropriate clinical practice. Evaluating the combination of CSF and blood PSP is also useful for distinguishing whether the complex central nervous system or systemic focus infection is involved.
Data availability statement
Data requests should be made to the corresponding author.
Ethical approval
The research/study was approved by the Institutional Review Board at Nara Prefecture General Medical Center, number 517, dated March 13, 2020.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abudeev SA, Kiselev KV, Kruglyakov NM, Belousova KA, Lobanova IN, Parinov OV. Cerebrospinal fluid presepsin as a marker of nosocomial infections of the central nervous system: A prospective observational study. Front Neurol. 2018. 9: 58
2. Bookland MJ, Sukul V, Connolly PJ. Use of a cyanoacrylate skin adhesive to reduce external ventricular drain infection rates. J Neurosurg. 2014. 121: 189-94
3. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012. 7: 47-8
4. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008. 36: 309-32
5. Hussein K, Bitterman R, Shofty B, Paul M, Neuberger A. Management of post-neurosurgical meningitis: Narrative review. Clin Microbiol Infect. 2017. 23: 621-8
6. Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009. 9: 245-55
7. Kondo Y, Umemura Y, Hayashida K, Hara Y, Aihara M, Yamakawa K. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: A systematic review and meta-analysis. J Intensive Care. 2019. 7: 22
8. Leen WG, Willemsen MA, Wevers RA, Verbeek MM. Cerebrospinal fluid glucose and lactate: Age-specific reference values and implications for clinical practice. PLoS One. 2012. 7: e42745
9. Li Z, Wu X, Yu J, Wu X, Du Z, Sun Y. Empirical combination antibiotic therapy improves the outcome of nosocomial meningitis or ventriculitis in neuro-critical care unit patients. Surg Infect (Larchmt). 2016. 17: 465-72
10. Mussap M, Noto A, Fravega M, Fanos V. Soluble CD14 subtype presepsin (sCD14-ST) and lipopolysaccharide binding protein (LBP) in neonatal sepsis: New clinical and analytical perspectives for two old biomarkers. J Matern Fetal Neonatal Med. 2011. 24: 12-4
11. Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001. 310: 173-86
12. Reiber H, Peter JB. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J Neurol Sci. 2001. 184: 101-22
13. Reichert MC, Medeiros EA, Ferraz FA. Hospital-acquired meningitis in patients undergoing craniotomy: Incidence, evolution, and risk factors. Am J Infect Control. 2002. 30: 158-64
14. Sandquist M, Wong HR. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev Clin Immunol. 2014. 10: 1349-56
15. Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011. 17: 764-9
16. Stubljar D, Kopitar AN, Groselj-Grenc M, Suhadolc K, Fabjan T, Skvarc M. Diagnostic accuracy of presepsin (sCD14-ST) for prediction of bacterial infection in cerebrospinal fluid samples from children with suspected bacterial meningitis or ventriculitis. J Clin Microbiol. 2015. 53: 1239-44
17. Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM. 2017 Infectious diseases society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017. 64: e34-65
18. van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010. 362: 146-54
19. Viallon A, Botelho-Nevers E, Zeni F. Clinical decision rules for acute bacterial meningitis: Current insights. Open Access Emerg Med. 2016. 8: 7-16
20. Yamamoto T, Nishimura T, Kaga S, Uchida K, Tachibana Y, Esaki M. Diagnostic accuracy of presepsin for sepsis by the new Sepsis-3 definitions. Am J Emerg Med. 2019. 37: 1936-41
21. Zheng G, Zhang C, Zhang G, Shao C. Evaluation of the diagnostic and prognostic value of CSF presepsin levels in patients with postneurosurgical ventriculitis/meningitis. Infect Drug Resist. 2021. 14: 2901-9