- Department of Neurological Surgery, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, 2-5-1 Shikata, Kita-ku, Okayama 700-8558, Japan
Correspondence Address:
Kazuhiko Kurozumi
Department of Neurological Surgery, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, 2-5-1 Shikata, Kita-ku, Okayama 700-8558, Japan
DOI:10.4103/sni.sni_62_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Yoshihiro Otani, Kazuhiko Kurozumi, Joji Ishida, Masafumi Hiramatsu, Masahiro Kameda, Tomotsugu Ichikawa, Isao Date. Combination of the tubular retractor and brain spatulas provides an adequate operative field in surgery for deep-seated lesions: Case series and technical note. 01-Nov-2018;9:220
How to cite this URL: Yoshihiro Otani, Kazuhiko Kurozumi, Joji Ishida, Masafumi Hiramatsu, Masahiro Kameda, Tomotsugu Ichikawa, Isao Date. Combination of the tubular retractor and brain spatulas provides an adequate operative field in surgery for deep-seated lesions: Case series and technical note. 01-Nov-2018;9:220. Available from: http://surgicalneurologyint.com/surgicalint-articles/9067/
Abstract
Background:Surgeries for deep-seated lesions are challenging because making a corridor and observing the interface between lesions and normal brain tissue are difficult. The ViewSite Brain Access System, which is a clear plastic tubular retractor system, is used for resection of deep-seated lesions. However, the tapered shape of this system may result in limitation of the surgical field and cause brain injury to observe the interface between lesions and normal tissue. In this study, we evaluated the usefulness of the combination of ViewSite and brain spatulas.
Methods:Nine patients were retrospectively identified who underwent resection of deep-seated lesions with the combination of Viewsite and brain spatulas. We assessed the extent of resection, prognosis, and quantitative brain injury from postoperative diffusion-weighed imaging (DWI).
Results:There were four total radiographically confirmed resections. Subtotal resection in four patients and partial resection in one with central neurocytoma were achieved because these tumors were strongly adherent to the choroid plexus and ependymal veins. Only one case of metastatic tumor relapsed 6 months after surgery. The mean postoperative high signal on DWI was 3.68 ± 0.80 cm3.
Conclusions:The combination of ViewSite and brain spatulas provides wide and adequate operative fields to observe the interface between lesions and normal tissue, and to prevent brain injury from excessive retraction pressure on the brain derived from repositioning of the ViewSite. Postoperative 3D volumetric analysis shows minimal damage to normal brain tissue. This report may provide new insight into the use of the ViewSite tubular retractor.
Keywords: Brain spatula, deep-seated lesion, tubular retractor, ViewSite
INTRODUCTION
Surgeries for intracranial deep-seated lesions are challenging, despite advancement and improvement of surgical approaches and techniques. Creation and maintenance of a safe corridor into the tumor is critical, as well as the ability to visualize the tumor and surrounding structures during the operation.[
Recently, the ViewSite Brain Access System (Vycor Medical, Inc., Boca Raton, FL, USA) has been used for resection of deep-seated lesions. The usefulness of this tubular retractor system for brain tumors has been documented in adult[
Figure 1
The ViewSite brain retractor. The ViewSite brain retractor is designed as a tapered shape to minimalize disruption to the brain during insertion (a). The width of the forward edge (solid line) is smaller than that of the reverse edge (dashed line) and it leads to limitation in the working space (b)
In this study, we report nine cases of deep-seated lesions, which were resected using the ViewSite tubular retractor and brain spatulas. We analyzed postoperative 3D volumetric changes in diffusion-weighted imaging (DWI).
MATERIALS AND METHODS
Patients
Patients with intracranial deep-seated lesions who were treated using both the ViewSite tubular retractor and brain spatulas were identified from the medical records of Okayama University Hospital from March 2013 to October 2016. Medical charts were reviewed for age at presentation, sex, clinical presentation, radiographic imaging, operation record, pathological findings, treatment, and outcome. This study (no. 1703-011) was approved by the ethical committee of the Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.
Surgical techniques
Preoperatively, for the neuronavigation system, computed tomography (CT) imaging and contrast-enhanced magnetic resonance imaging (MRI) were performed. When the lesion was adjacent to the eloquent area, functional MRI and diffusion tensor imaging (DTI) were also achieved. Using these images, either Curve™ Image Guided Surgery (BrainLab, Munich, Germany), StealthStation S7 (Medtronic Inc., Louisville, CO, USA), or StealthStation Treon (Medtronic Inc.) was registered, and the surgical trajectory was planned to avoid the eloquent area and critical vasculature. After corticotomy was performed over a non-eloquent gyrus, the ViewSite tubular retractor was gently inserted along the previously planned surgical tract. As mentioned above, there are several sizes of the ViewSite tubular retractor. Therefore, the distance from the entry point of parenchyma to the lesion was measured on images to decide which retractor was suitable. We usually selected 17- and 21-mm-wide retractors in the 7 cm lengths. After setting the ViewSite tubular retractor in the proper position, the retractor was fixed with a self-retaining Leyla arm (Aesculap, Tuttlingen, Germany), and the introducer was removed. We then resected lesions through the ViewSite tubular retractor under a surgical microscope (OPMI Pentero or NC4; Carl Zeiss Co., Oberkochen, Germany) and/or an endoscope (Karl Storz Endoscopy, Tuttlingen, Germany) [Figure
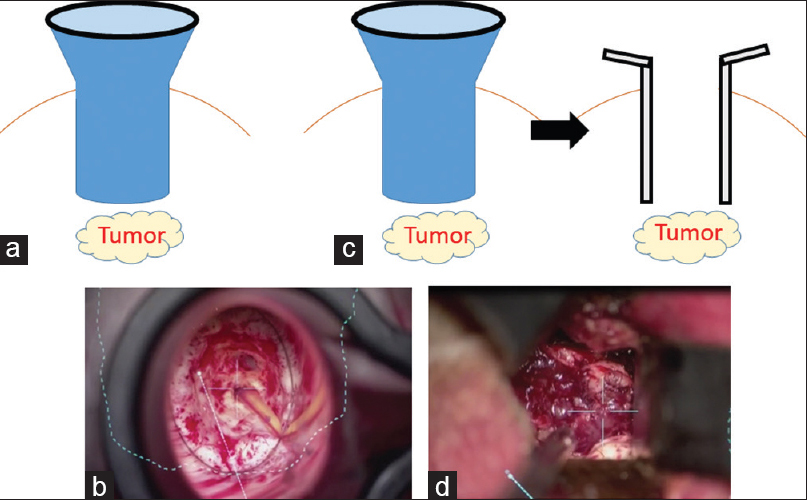
Figure 2
Illustration depicting surgical approaches to deep-seated lesions with the ViewSite tubular retractor or brain spatulas. When only using the ViewSite tubular retractor, the surgical field is limited because of its tapered shape (a and b). However, using brain spatulas after making a corridor to the lesion with the ViewSite tubular retractor provides an adequate surgical field (c and d)
At the end of resection, a ventriculostomy catheter was placed at the intraventricular space in ventricular tumor surgery. Finally, the dura mater, the bone flap, and skin were closed in a standard fashion.
Volumetric analysis
Researcher who was not involved in the surgical procedure of the patients in this study conducted the volumetric analysis described below. Postoperative radiographic MRI was imported into BrainLab software for volumetric analysis. DWI (b-value; 1000) was used because restricted diffusion on DWI is a marker for brain cytotoxic edema and ischemic or cell damage.[
RESULTS
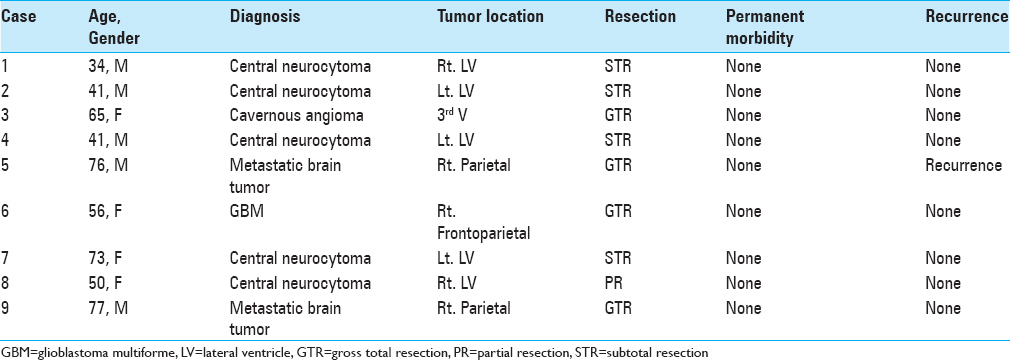
A comprehensive list of the patients is shown in
Illustrative case
A 56-year-old woman was transferred to our hospital with sensory disturbance in her left arm and leg. MRI of the head showed a 29-mm tumor in the right frontal and parietal lobes. The tumor had a necrotic area in the center of the tumor. After administration of contrast enhancement, the tumor showed ring enhancement [Figure
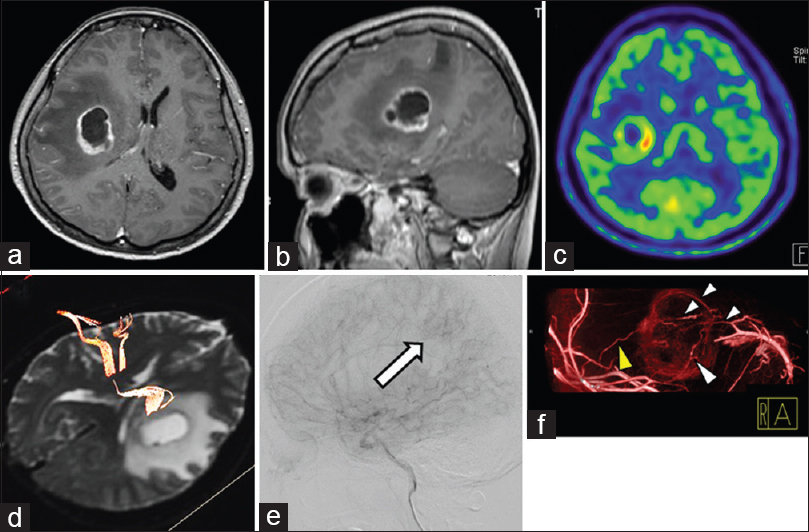
Figure 3
Preoperative imaging of an illustrative case. Preoperative contrast-enhanced axial (a) and sagittal (b) MRI shows a 29-mm tumor in the right frontal and parietal lobes. 11C-methionine positron emission tomography shows high uptake (c), and DTI shows that the corticospinal tract is present in the front of and adjacent to the tumor itself (d). Digital subtraction angiogram shows tumor staining (e, arrow). Contrast-enhanced cone-beam CT shows that main feeding arteries (yellow arrowhead) from the right middle cerebral artery are located in the lateral part of the tumor, and draining veins (white arrowhead) are in the medial portion of the tumor (f)
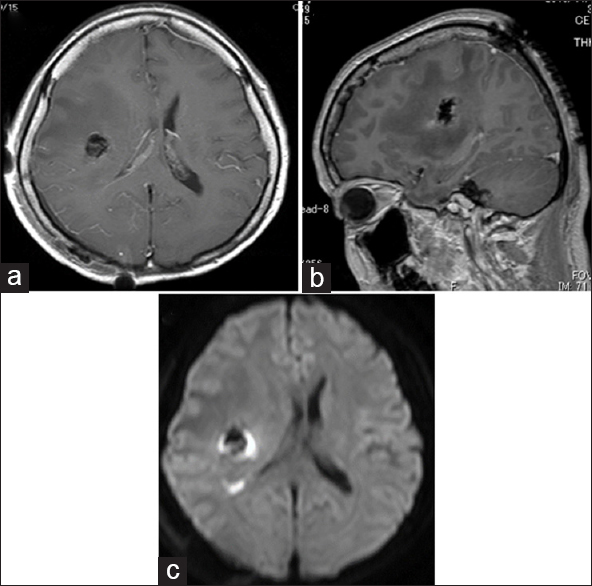
Postoperative MRI showed GTR [Figure
DISCUSSION
Surgeries for deep-seated lesions are challenging because creating a corridor and observing the interface between lesions and normal brain tissue are difficult. To solve these problems, many brain retraction systems combined with a microscope or endoscope have been introduced. First, the self-retaining retraction system by Greenberg was introduced in 1981.[
The ViewSite tubular retractor is a specially tailored tubular retractor for neurosurgery and applied for removal of intraparenchymal or intraventricular tumors, hemorrhage, and foreign bodies.[
The concept of tubular retractor was proposed more than 30 years ago, and their efficacy and safety have been reported numerously compared with traditional blade retractors that may cause asymmetric tension on brain tissue. By contrast, we also feel the disadvantages of tubular retractor, which are the limitation of working space and observing irregular border of deep-seated lesions. The brain spatulas compensate these disadvantages, and we can provide minimally invasive surgery by using the combination of the ViewSite tubular retractor and brain spatulas technique.
CONCLUSION
In conclusion, we first introduced the combination of the ViewSite tubular retractor and brain spatulas to overcome the disadvantage of the ViewSite tubular retractor, including the limitation in the area and view of the operative field. Our results may provide new insight into the use of the ViewSite tubular retractor.
Financial support and sponsorship
Nil.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
1. Albin MS, Bunegin L. The insidiousness of brain retractor pressure: Another “Smoking gun”?. AnesthAnalg. 2003. 96: 306-
2. Andrews RJ, Bringas JR. A review of brain retraction and recommendations for minimizing intraoperative brain injury. Neurosurgery. 1993. 33: 1052-
3. Bander ED, Jones SH, Kovanlikaya I, Schwartz TH. Utility of tubular retractors to minimize surgical brain injury in the removal of deep intraparenchymal lesions: A quantitative analysis of FLAIR hyperintensity and apparent diffusion coefficient maps. JNeurosurg. 2016. 124: 1053-60
4. Bernardo A, Evins AI, Tsiouris AJ, Stieg PE. A Percutaneous transtubular middle fossa approach for intracanalicular tumors. World Neurosurg. 2015. 84: 132-46
5. Fahim DK, Relyea K, Nayar VV, Fox BD, Whitehead WE, Curry DJ. Transtubular microendoscopic approach for resection of a choroidal arteriovenous malformation. JNeurosurg Pediatr. 2009. 3: 101-4
6. Greenberg I. Self-retaining retractor and handrest system for neurosurgery. Neurosurgery. 1981. 8: 205-8
7. Greenfield JP, Cobb WS, Tsouris AJ, Schwartz TH. Stereotactic minimally invasive tubular retractor system for deep brain lesions. Neurosurgery. 2008. 63: 334-
8. Harada S, Nakamura T. Retraction induced brain edema. Acta Neurochir Suppl (Wien). 1994. 60: 449-51
9. Herrera SR, Shin JH, Chan M, Kouloumberis P, Goellner E, Slavin KV. Use of transparent plastic tubular retractor in surgery for deep brain lesions: A case series. SurgTechnol Int. 2010. 19: 47-50
10. Ichikawa T, Otani Y, Ishida J, Fujii K, Kurozumi K, Ono S. Hybrid microscopic-endoscopic surgery for craniopharyngioma in neurosurgical suite: Technical notes. World Neurosurg. 2016. 85: 340-8.e1
11. Kassam AB, Engh JA, Mintz AH, Prevedello DM. Completely endoscopic resection of intraparenchymal brain tumors. JNeurosurg. 2009. 110: 116-23
12. Kelly PJ. Future perspectives in stereotactic neurosurgery: Stereotactic microsurgical removal of deep brain tumors. JNeurosurg Sci. 1989. 33: 149-54
13. Kishida Y, Sato T, Oda K, Ichikawa M, Sakuma J, Saito K. Pure endoscopic resection of deep intracranial tumors using the ViewSite Brain Access System. No shinkei geka. 2014. 42: 311-25
14. Matsumoto Y, Kurozumi K, Shimazu Y, Ichikawa T, Date I. Endoscope-assisted resection of cavernous angioma at the foramen of Monro: A case report. Springerplus. 2016. 5: 1820-
15. Ogura K, Tachibana E, Aoshima C, Sumitomo M. New microsurgical technique for intraparenchymal lesions of the brain: Transcylinder approach. Acta Neurochir (Wien). 2006. 148: 779-85
16. Recinos PF, Raza SM, Jallo GI, Recinos VR. Use of a minimally invasive tubular retraction system for deep-seated tumors in pediatric patients. JNeurosurg Pediatr. 2011. 7: 516-21
17. Rosenorn J, Diemer NH. Reduction of regional cerebral blood flow during brain retraction pressure in the rat. JNeurosurg. 1982. 56: 826-9
18. Rymarczuk GN, Davidson L, Severson MA, Armonda RA. Use of a minimally invasive retractor system for retrieval of intracranial fragments in wartime trauma. World Neurosurg. 2015. 84: 1055-61
19. Shoakazemi A, Evins AI, Burrell JC, Stieg PE, Bernardo A. A 3D endoscopic transtubular transcallosal approach to the third ventricle. JNeurosurg. 2015. 122: 564-73
20. Yadav YR, Yadav S, Sherekar S, Parihar V. A new minimally invasive tubular brain retractor system for surgery of deep intracerebral hematoma. Neurol India. 2011. 59: 74-7







Roberto Martínez alvarez
Posted November 30, 2018, 11:58 pm
Excellent idea, please how I can get this device?
Thank you very much
Kazuhiko Kurozumi
Posted February 3, 2019, 8:51 am
Thank you very much for your comment. I didn’t notice your comments. Please ask the company which I wrote to know where you can get it.