- Department of Neurosurgery, Neurological Institute of Thailand,
- Department of Neuroradiology, Neurological Institute of Thailand,
- Department of Radiology, Bumrungrad International Hospital, Bangkok, Thailand.
Correspondence Address:
Prasert Iampreechakul, Department of Neurosurgery, Neurological Institute of Thailand, 312, Rachawithi Road, Khwaeng Thung Phaya Thai, Bangkok, 10400, Thailand.
DOI:10.25259/SNI_308_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prasert Iampreechakul1, Anusak Liengudom1, Wuttipong Tirakotai1, Korrapakc Wangtanaphat1, Punjama Lertbutsayanukul2, Sunisa Hangsapruek2, Somkiet Siriwimonmas3. Combined endovascular and microsurgical management of complex traumatic carotid-cavernous fistula: Three case reports. 05-Aug-2022;13:337
How to cite this URL: Prasert Iampreechakul1, Anusak Liengudom1, Wuttipong Tirakotai1, Korrapakc Wangtanaphat1, Punjama Lertbutsayanukul2, Sunisa Hangsapruek2, Somkiet Siriwimonmas3. Combined endovascular and microsurgical management of complex traumatic carotid-cavernous fistula: Three case reports. 05-Aug-2022;13:337. Available from: https://surgicalneurologyint.com/surgicalint-articles/11771/
Abstract
Background: With the evolution of the endovascular devices, the management of endovascular interventions has become the current standard therapy for traumatic carotid-cavernous fistula (TCCF). However, only endovascular treatment may not be feasible in some patients with atypical TCCF.
Case Description: We described three complex TCCFs that could not be managed by conventional endovascular methods. The first patient had recurrent TCCF previously treated by muscle embolization and ligation of affected carotid arteries 23 years ago. Another two patients had TCCFs association with large pseudoaneurysm within the sphenoid sinus. In each patient, the fistula was successfully closed by trapping procedure using a combination of endovascular and surgical treatment.
Conclusion: To reduce costs of treatment, trapping operation by combining surgical and endovascular treatment may be considered as an alternative option for complex TCCF which has some features including chronic stage, preexisting carotid artery ligation, or association with large venous pouch of the cavernous sinus or sphenoid sinus pseudoaneurysm.
Keywords: Chronic recurrent fistula, Direct carotid-cavernous fistula, Sphenoid sinus pseudoaneurysm, Trapping procedure, Traumatic carotid-cavernous fistula
INTRODUCTION
Direct carotid-cavernous fistulas (CCFs) are high-flow shunts directly communicated between the internal carotid artery (ICA) and the cavernous sinus, categorizing as Type A by Barrow classification of CCFs.[
There are two basic forms of therapy for traumatic carotid-cavernous fistula (TCCF) including obliteration of the fistula while maintaining patency of the ICA and sacrifice of the affected ICA.[
CASE DESCRIPTION
Case 1
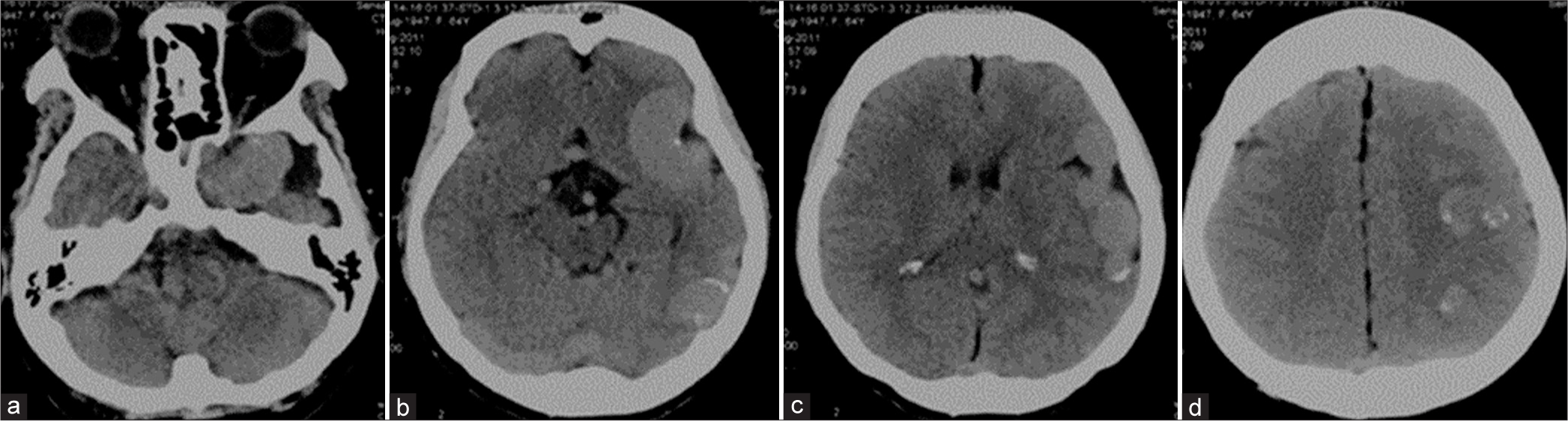
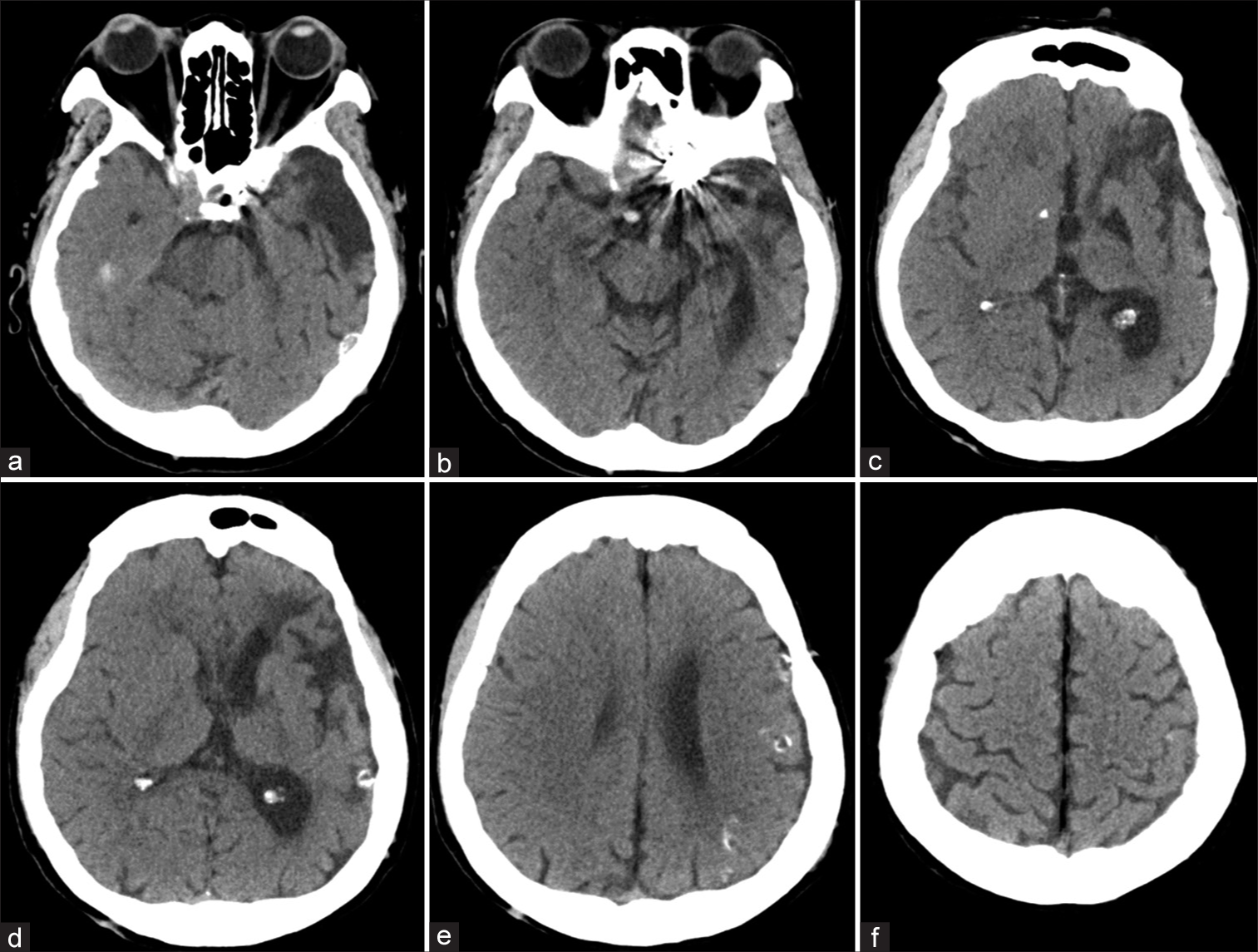
A 64-year-old left-handed woman was admitted to the local hospital due to seizure with transient loss of consciousness. She complained of headache and mild cognitive impairment for the past 1 year. A computed tomography (CT) scan of the head showed markedly dilatation of the left CS, sphenoparietal sinus, and cortical veins along left cerebral hemisphere. There was some venous wall calcification [
Figure 2:
Patient 1. Sequential (a-c) axial and (d) sagittal T2-weighted magnetic resonance images of the brain reveal markedly dilated left sphenoparietal sinus and cortical veins along left cerebral hemisphere. (e) Anteroposterior and (f) lateral views of magnetic resonance venographic images disclose multiple serpiginous engorged cortical veins in the left cerebral hemisphere.
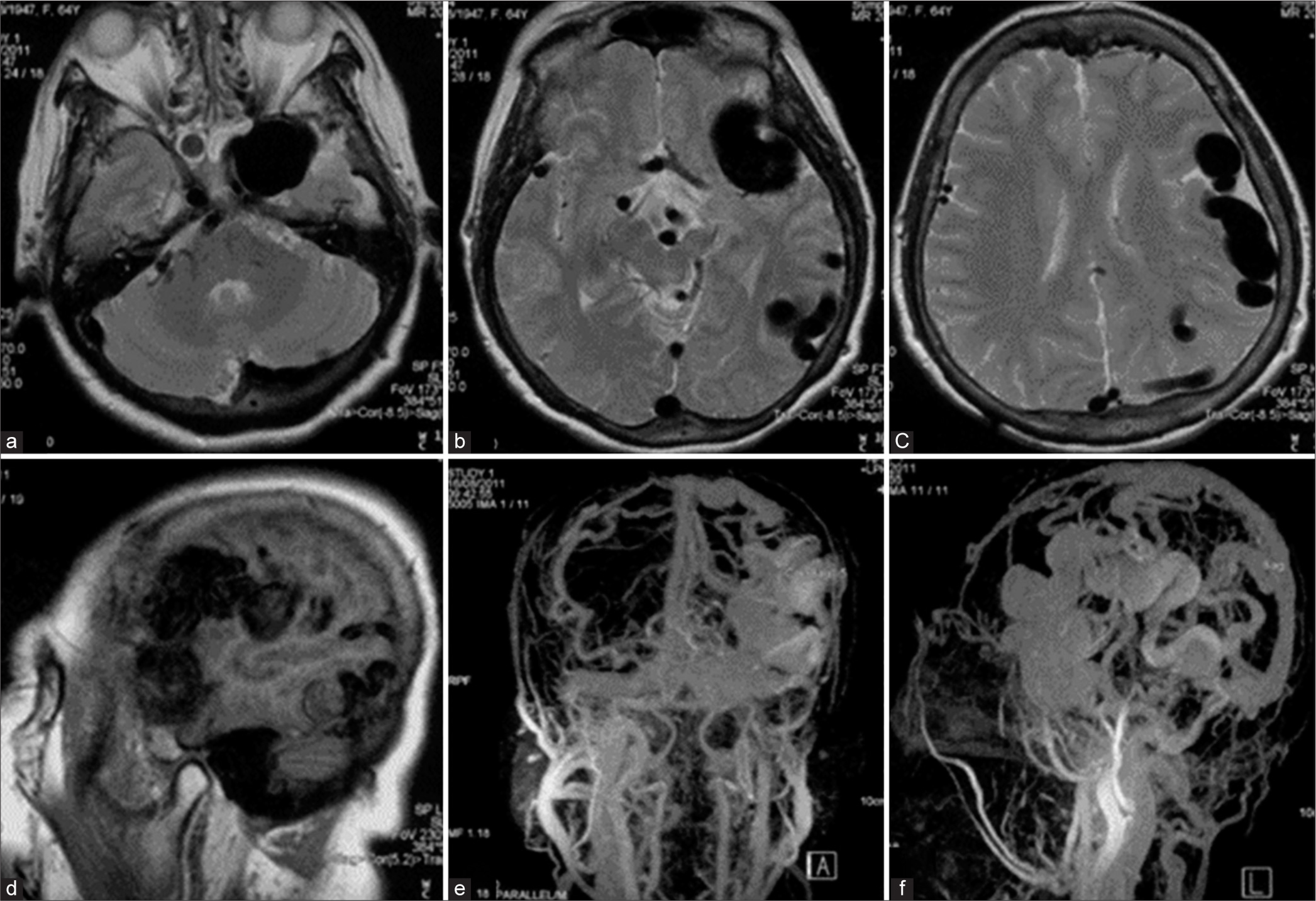
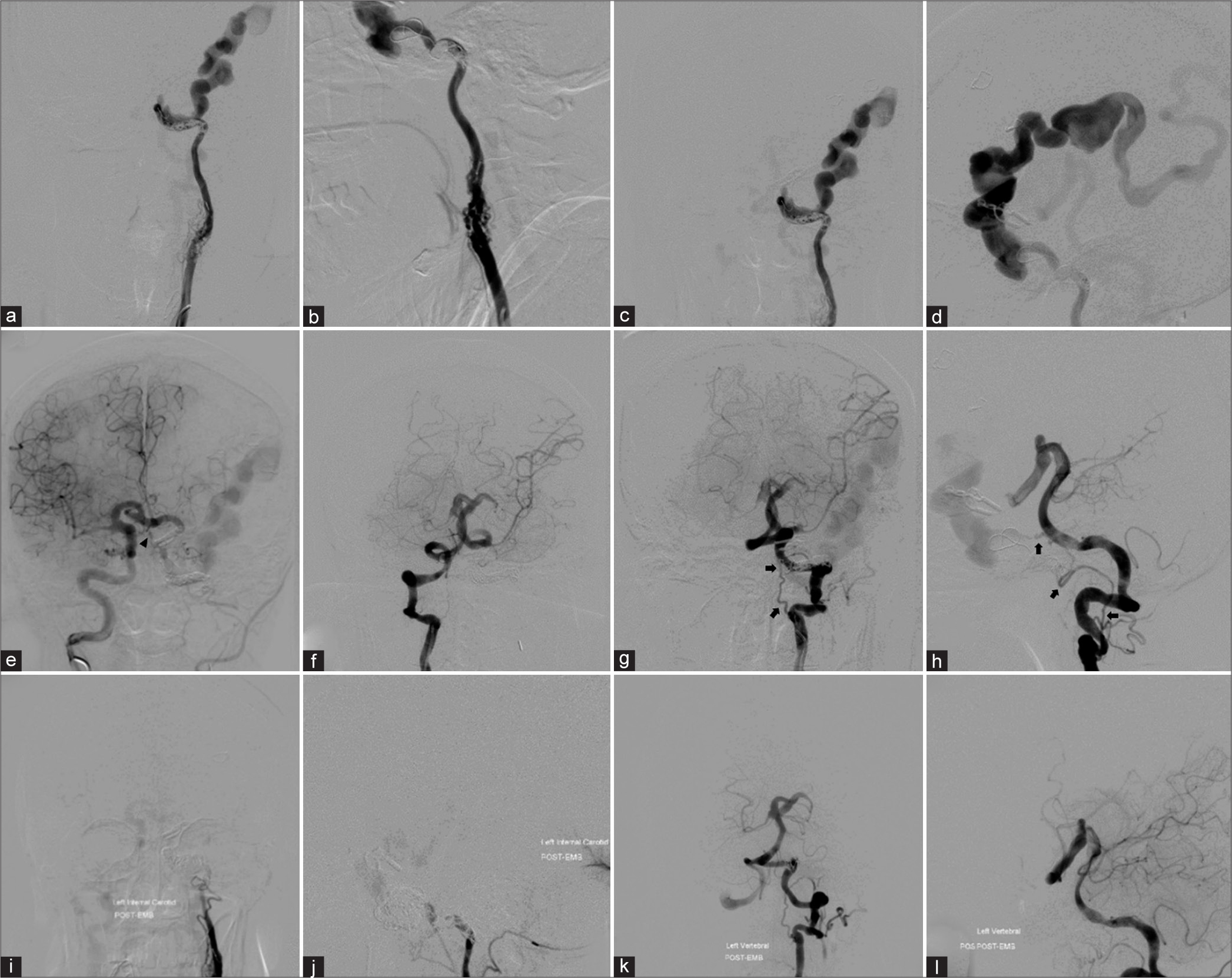
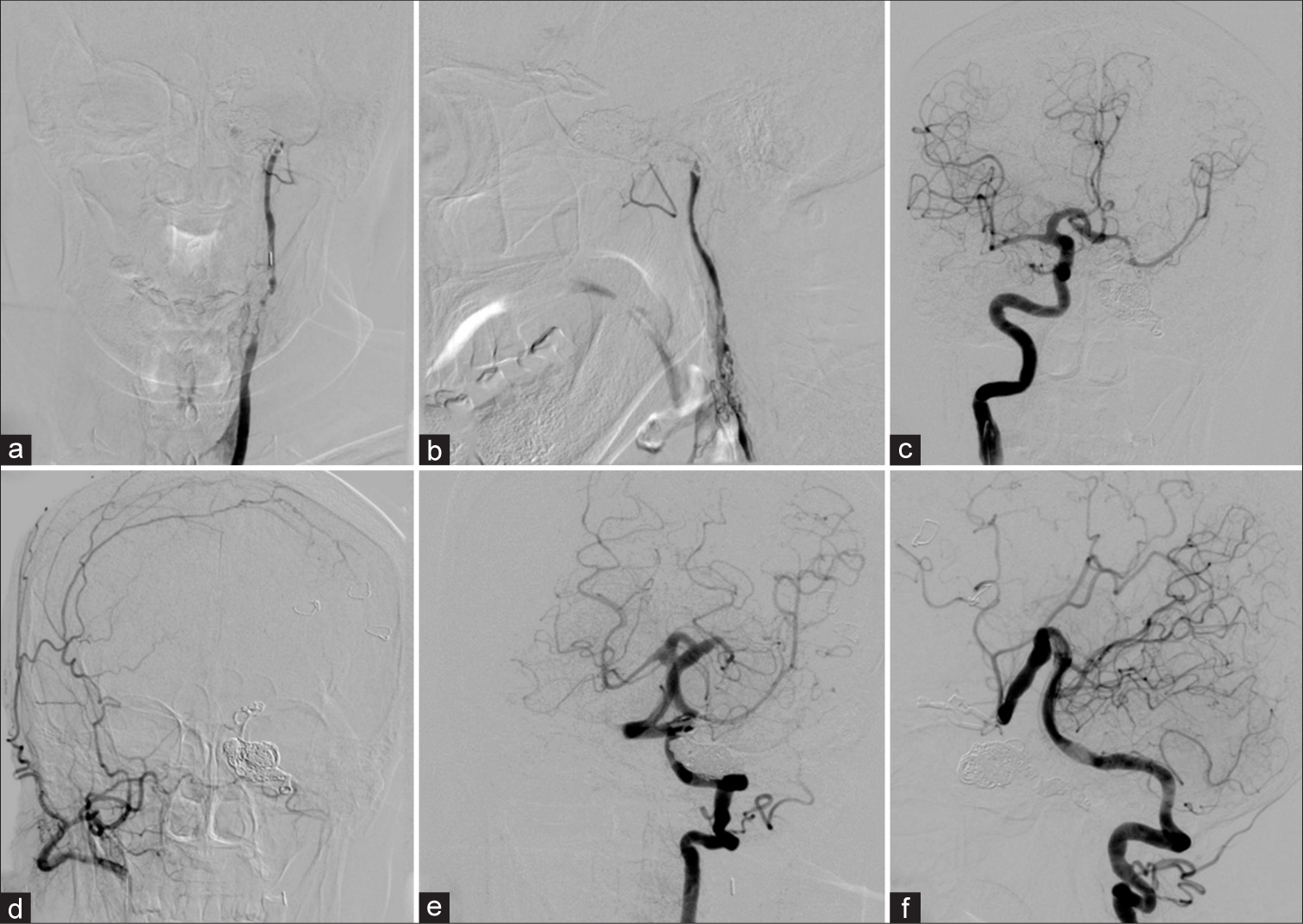
Cerebral angiography was performed before treatment. The left common carotid artery (CCA) injection with 3D rotational angiography showed no the existence of the external carotid artery (ECA), severe stenosis of the proximal ICA, and long segment of multiple small vascular channels, representing the recanalization of the left occluded ICA through vasa vasorum, at C3 to C4 vertebral level. The previous surgical clip is noted [
Figure 4:
Patient 1. (a) Anteroposterior (AP) and (b) lateral views with (c, d) 3D rotational angiography of the left common carotid artery injection show no the existence of the external carotid artery, severe stenosis of the proximal internal carotid artery (ICA), and long segment of multiple small vascular channels, representing the recanalization of the left ICA through vasa vasorum, at the level of C3–C4. Previous surgical clip (arrow) is noted. AP views of the (e) left and (i) right ICAs injections and lateral views of (f) the left ICA and the left vertebral artery injections in (g) arterial and (h) venous phases demonstrate the left direct carotid-cavernous fistula (arrowheads) with retrograde drainage into markedly engorged sphenoparietal sinus and superficial middle cerebral veins. Following the occlusion of the left petrous ICA with coils, (j) AP and (k) lateral views of the left ICA injection reveal significant reduction of shunt flow. (l) AP view of unsubtracted image illustrates the entire coil mesh.
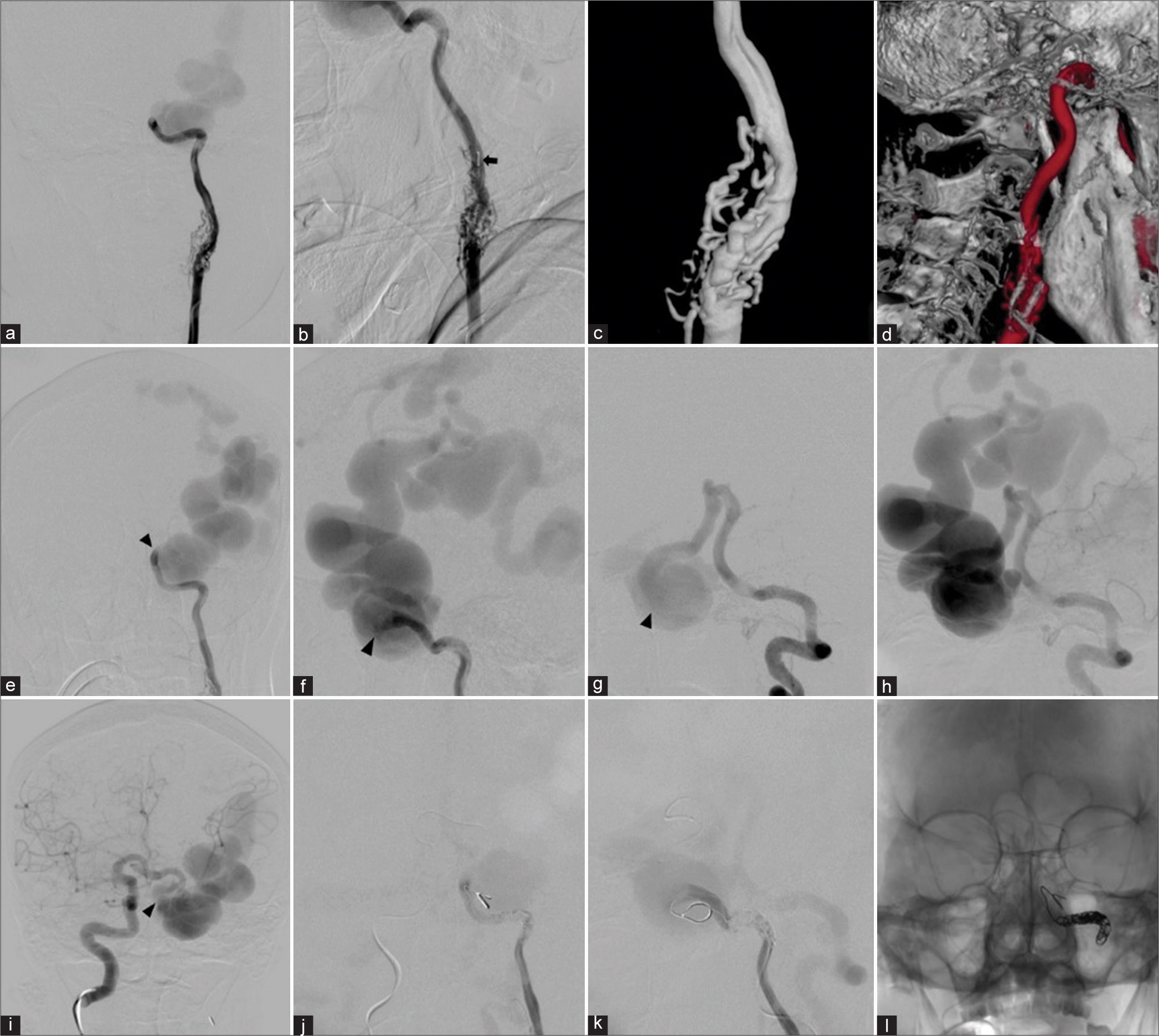
Under general anesthesia and heparinization, transarterial detachable balloon embolization was attempted, but the detachable balloon catheter could not be advanced into the fistula through ACoA from the right ICA, and PCoA from the VA. Then, the microcatheter was navigated into the fistula through ACoA from the right ICA. The largest GDC coil was attempted to place into the fistula, but the retrograde shunt into the fistula was so great that the coils mass was unable and kept migrating into the large venous pouch. Subsequently, the microcatheter was advanced further into the petrous segment of the left ICA proximal to the fistula which was occluded with Axium Detachable coil (Medtronic, Minneapolis, Minnesota, USA) [
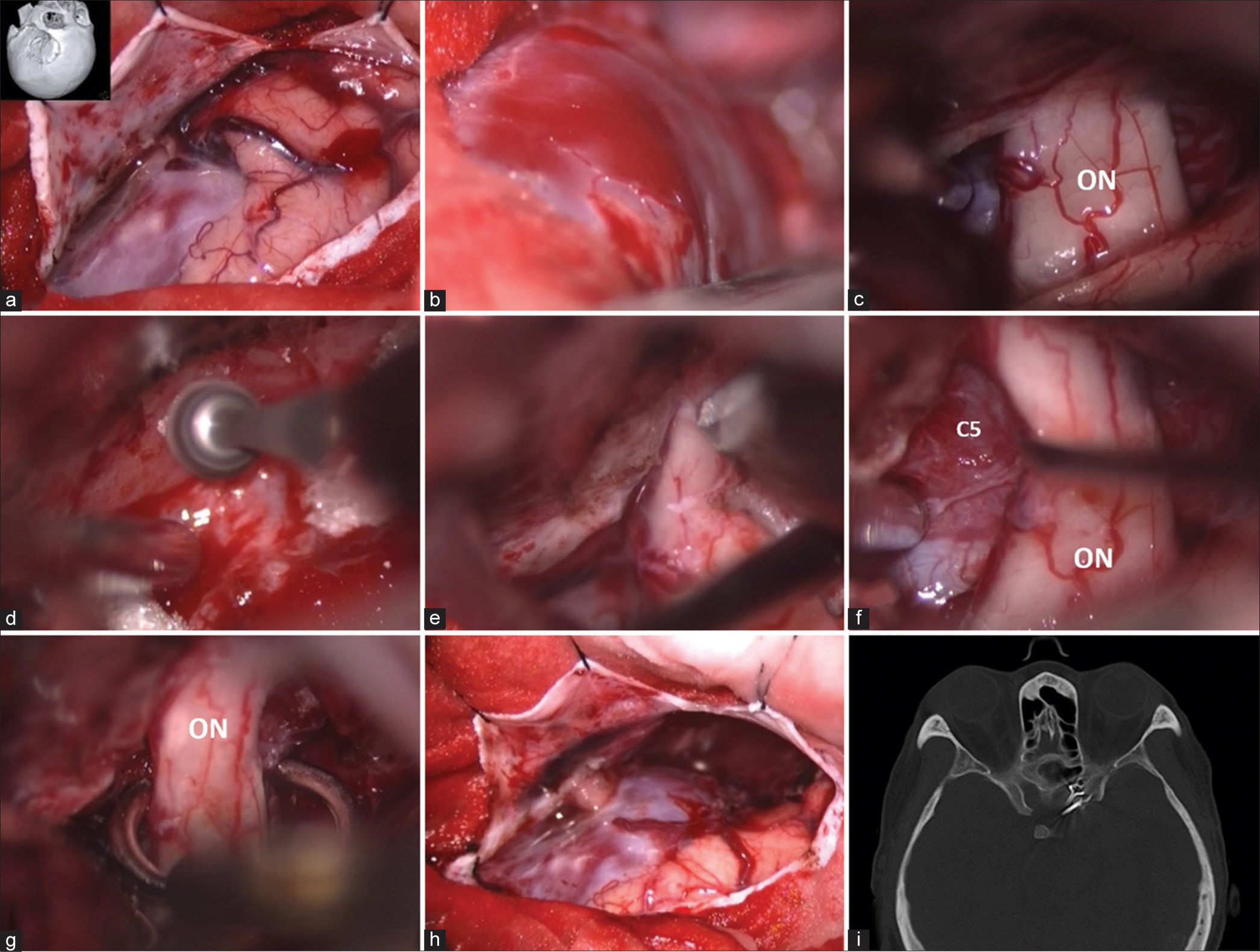
On the following day, the patient underwent the left frontotemporal craniotomy. Anterior clinoidectomy was subsequently performed, because it was difficult to place the clip on the ICA due to the large arterialized venous pouch. The clinoidal segment of the left ICA was clipped by the fenestrated clip encircling the left optic nerve [
Figure 5:
Patient 1. Intraoperative photographs through the left pterional approach. (a) After durotomy. (b) Arterialized venous pouch. (c) Identifying the left optic nerve (ON). (d) Removal of the left anterior clinoid process. (e) Opening the dura over the ON. (f) Identifying the clinoidal segment of the left internal carotid artery (C5) under the ON. (g) Applying the fenestrated clip encircling the ON. (h) Before closing the dura. (i) axial view of cranial computed tomography bone window scan demonstrates the surgical clips.
Figure 6:
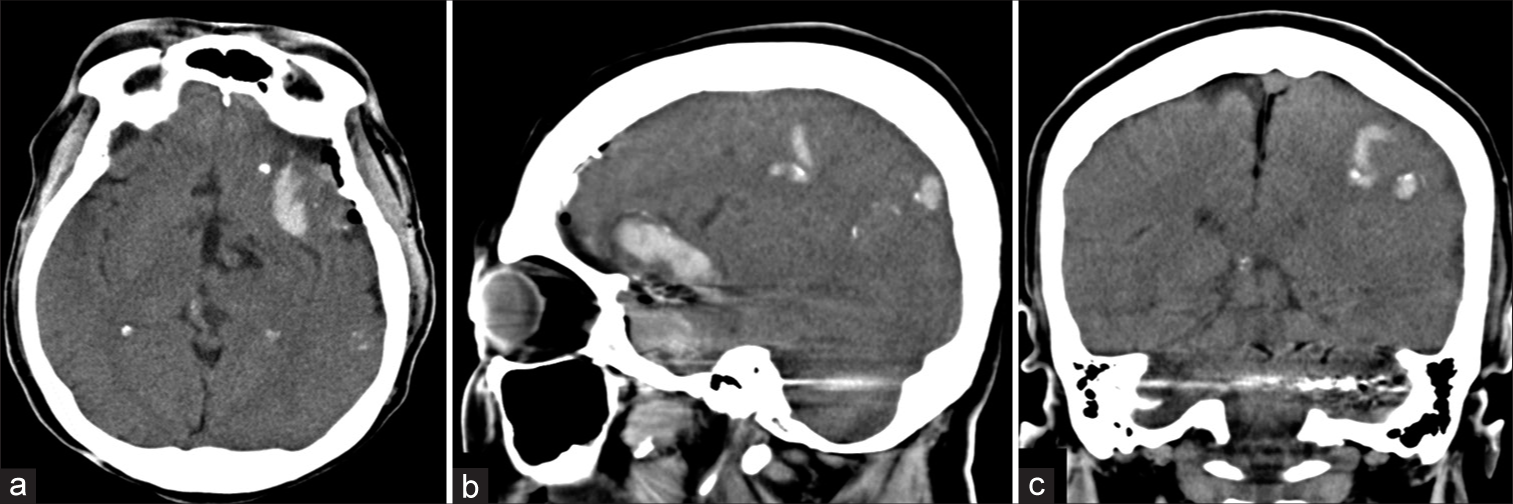
Patient 1. (a) axial, (b) sagittal, and (c) coronal images of noncontrast cranial computed tomography scan, obtained 3 h after surgery, reveal abnormal hyperdensity within dilated cortical veins along left cerebral hemisphere, probably representing partially thrombosis of the cerebral veins.
One month after surgery, cerebral angiography was obtained and revealed the remaining of severe stenosis of the proximal ICA with associated vasa vasorum and reduction in shunt flow and size of the dilated draining vein along the left cerebral hemisphere [
Figure 7:
Patient 1. Cerebral angiography obtained 1 month after surgery. (a-d) Anteroposterior (AP) and lateral views of the left common carotid artery injection reveals the remaining of severe stenosis of the proximal internal carotid artery (ICA) with associated vasa vasorum and reduction in shunt flow and size of the dilated draining vein along the left cerebral hemisphere. (e) AP view of the right ICA injection demonstrates the clival branch (arrowhead) from the right meningohypophyseal trunk supplying the fistula. AP views of the (f) right and (g) left vertebral arteries (VAs) and (h) lateral view of the left VA injections show the reconstitution from the muscular branch (arrows) of the left VA feeding the fistula. After embolization with coils at the fistulous part, (i) AP and (j) lateral views of the left CCA and (k) AP and (l) lateral views of the left VA confirm nearly complete obliteration of the fistula.
Figure 8:
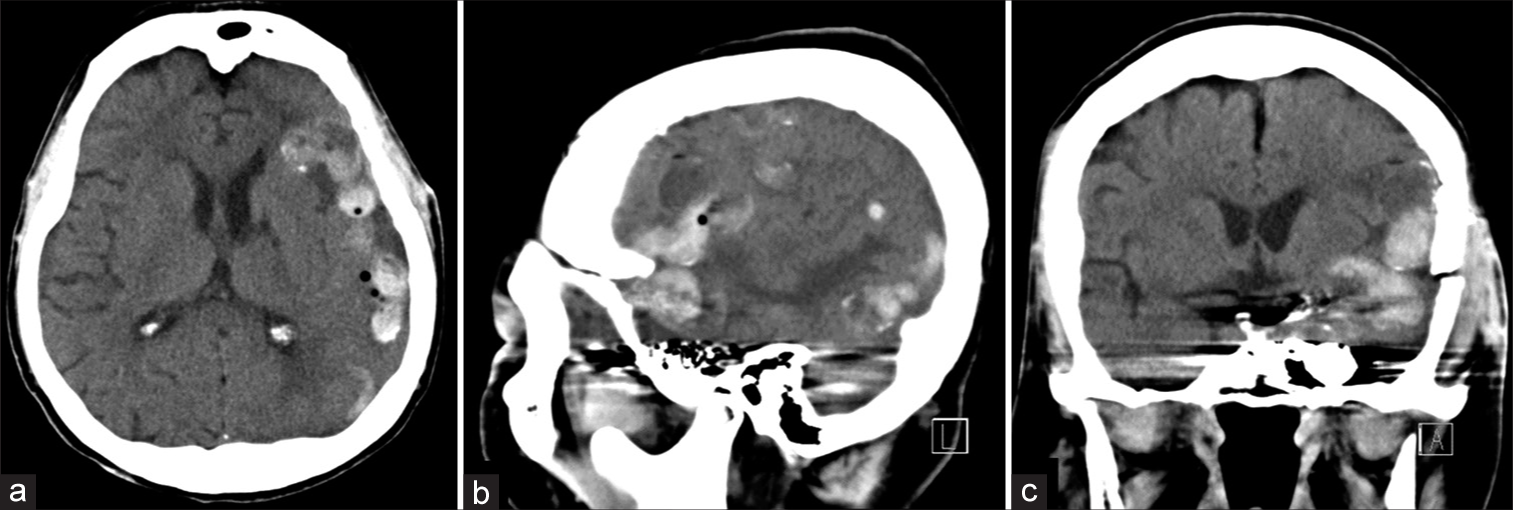
Patient 1. (a) axial, (b) sagittal, and (c) coronal images of noncontrast cranial computed tomography scan, obtained 5 days after second embolization with coils, reveal abnormal hyperdensity within dilated cortical veins along left cerebral hemisphere, probably representing ongoing venous thrombosis.
Figure 9:
Patient 1. Cerebral angiography obtained 1 year after the second embolization. (a) Anteroposterior (AP) and (b) lateral views of the left common carotid artery, (c) AP view of the right internal carotid artery, (d) AP view of the right external carotid artery, and (e) AP and (f) lateral views of the left vertebral artery injections confirm no recurrence of the fistula.
Case 2
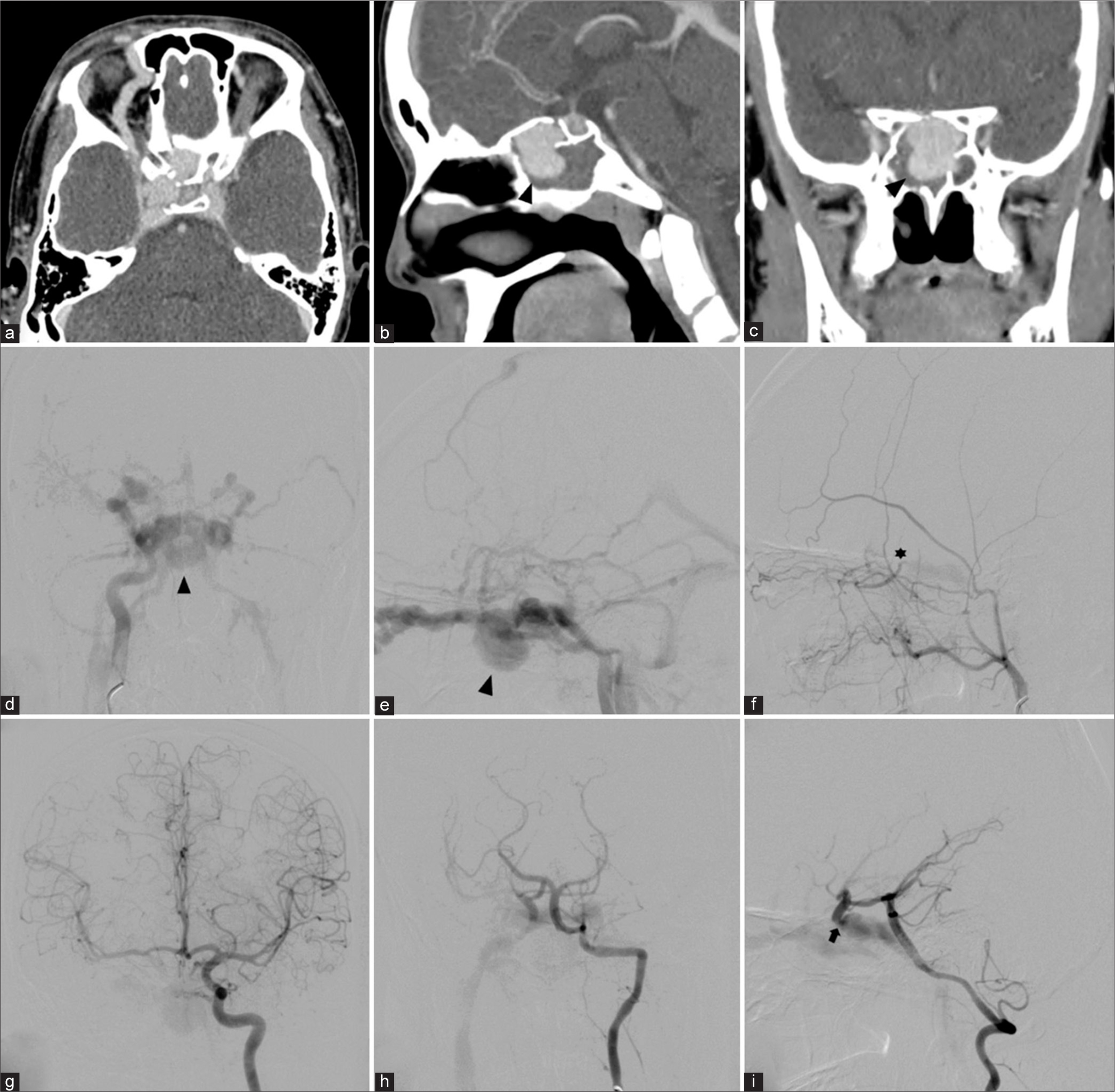
A 14-year-old right-handed boy sustained a severe head injury following a motorcycle accident with multiple organ injuries, including skull base fracture, right traumatic eye injury leading to blindness, left tympanic membrane perforation, and close fracture both bones right forearm. He was admitted to a local hospital and discharged home 3 weeks later. One month later, he developed right proptosis, bilateral audible bruit, and binocular horizontal diplopia. He went back to the previous local hospital and obtained cranial CT scan with contrast injection. The CT scan showed fractures at right-sided planum sphenoidale and superior wall of the sphenoid sinus, enlarged right superior ophthalmic vein (SOV), dilatation of bilateral CS, and large pseudoaneurysm within sphenoid sinus [
Figure 11:
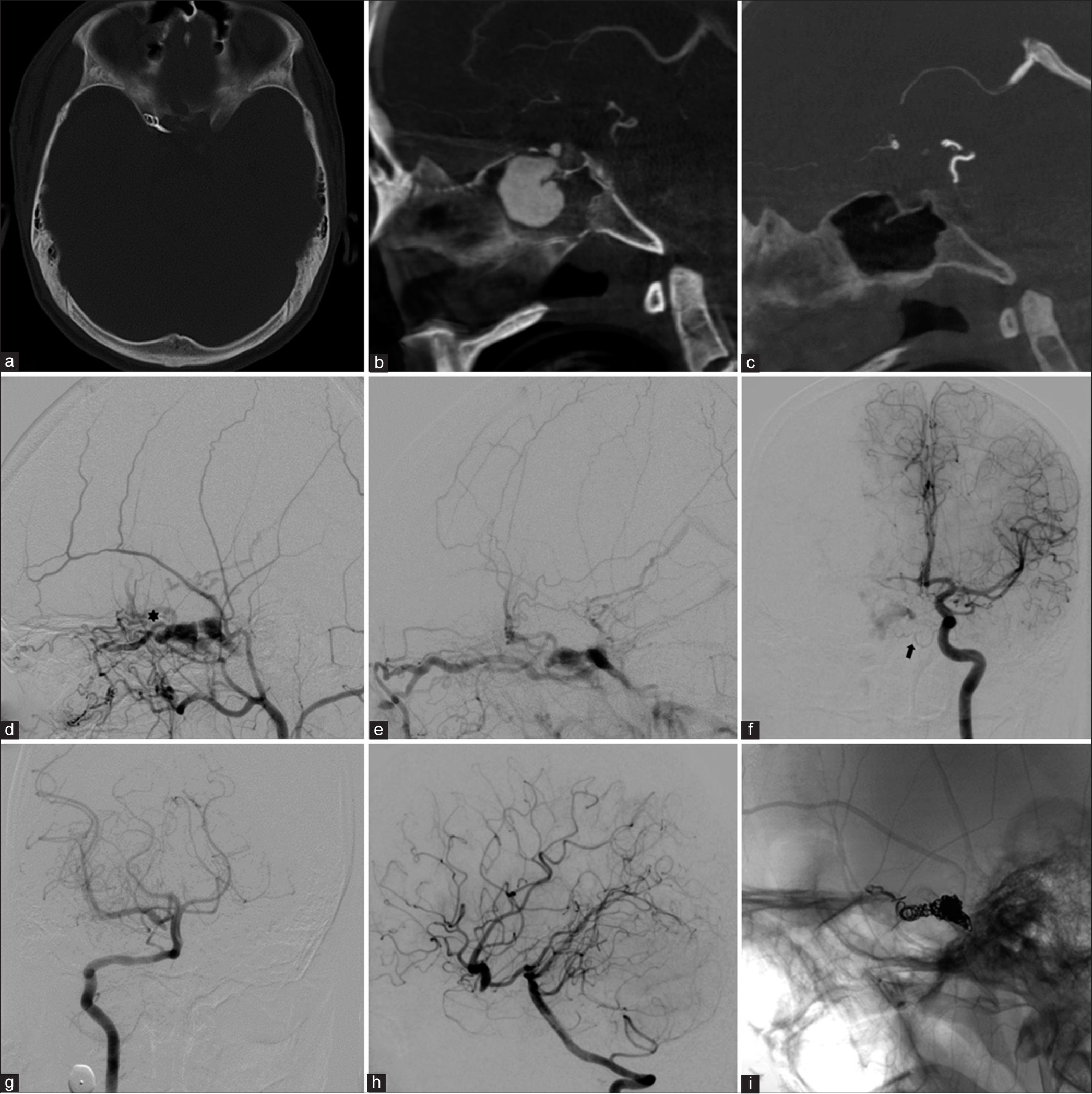
Patient 2. (a) Axial, (b) sagittal, and (c) coronal images of contrasted cranial computed tomography scan reveal skull base fracture, the dilatation of the right ophthalmic vein, and a large pseudoaneurysm (arrowhead) within the sphenoid sinus. (d) Anterolateral (AP) and (e) lateral views of the right internal carotid artery (ICA) show direct high-flow carotid-cavernous fistula with a large pseudoaneurysm (arrowhead). (f) Lateral view of the right external carotid artery demonstrates retrograde flow through the right ophthalmic artery (asterisk) through meningo-ophthalmic artery anastomosis, supplying the fistula. (g) AP view of the left ICA injection discloses the clival branch from the left meningohypophyseal trunk supplying the fistula. (h) AP and (i) lateral views of the left vertebral artery illustrate the fistulous point (arrow) between the clinoidal segment of the right ICA and cavernous sinus.
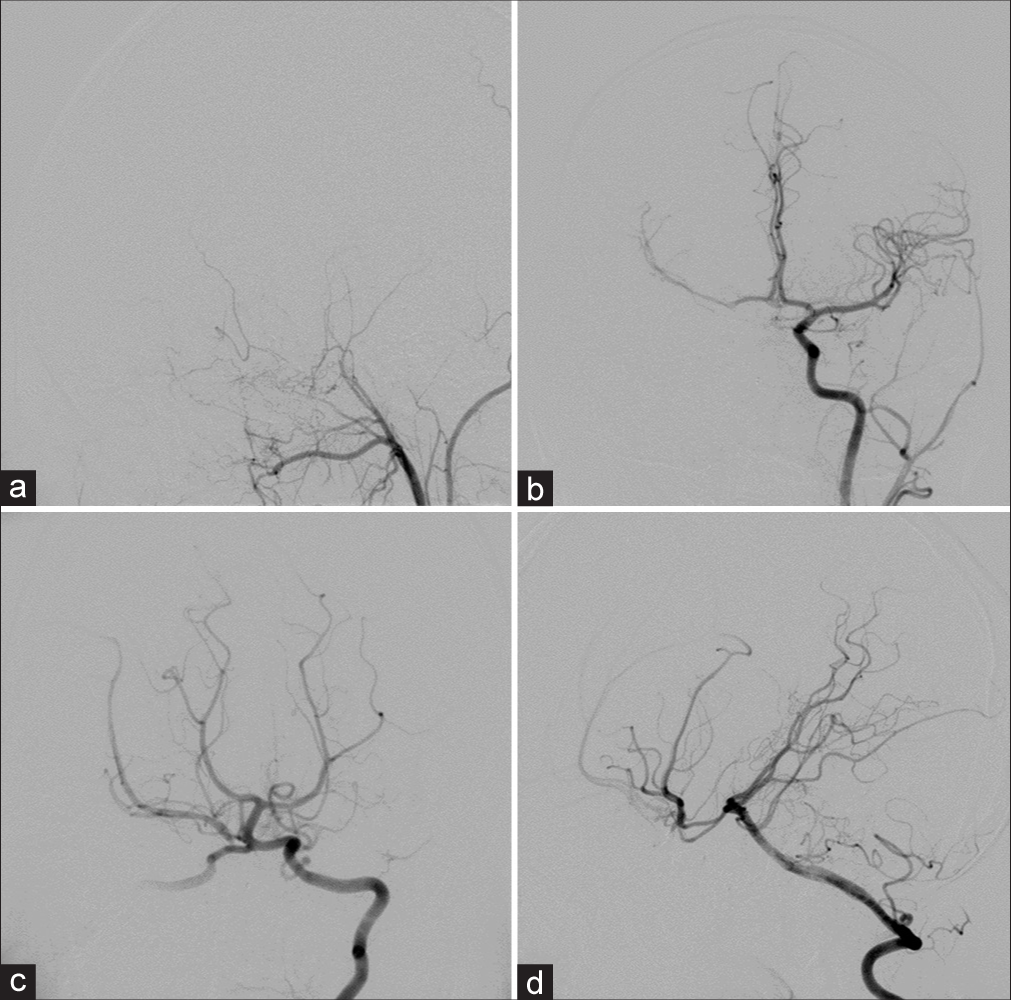
Cerebral angiography was performed and revealed direct high-flow fistula between clinoidal segment of the right ICA and dilated right CS with a large pseudoaneurysm arising from medial aspect of the right clinoidal ICA and extending inferiorly into sphenoid sinus. The right ICA terminated in the fistula, and there was no filling of the distal carotid artery. The large pseudoaneurysm located just proximal to the site of CCF. Multiple venous drainages included right SOV, right SMCV, right inferior petrosal sinus (IPS), right basal vein of Rosenthal to the vein of Galen, and posterior intercavernous sinus draining into the left CS. The subsequent venous drainages from the left CS ran anteriorly into the left SOV, and posteroinferiorly into the left IPS [
Under general anesthesia and heparinization, transarterial detachable balloon embolization was attempted to place to balloon across the orifice of the fistula, but the balloon always migrated into the pseudoaneurysm. It was impossible to place a balloon in a stable position across the orifice of the fistula, and we were unable to navigate either a balloon or microcatheter beyond the fistula to allow distal control. Therefore, the right ICA was occluded with detachable balloons at two points including just proximal to the fistula and at the cervical portion of the right ICA. The right CCA injection confirmed complete occlusion of the right ICA with intradural ICA opacification through retrograde filling of the right meningo-OA anastomosis [
Figure 12:
Patient 2. (a) Anteroposterior and (b) lateral views of the right common carotid artery confirm complete occlusion of the right carotid artery after the occlusion of detachable balloons with intradural internal carotid artery opacification through retrograde filling of the right meningo-ophthalmic artery anastomosis.
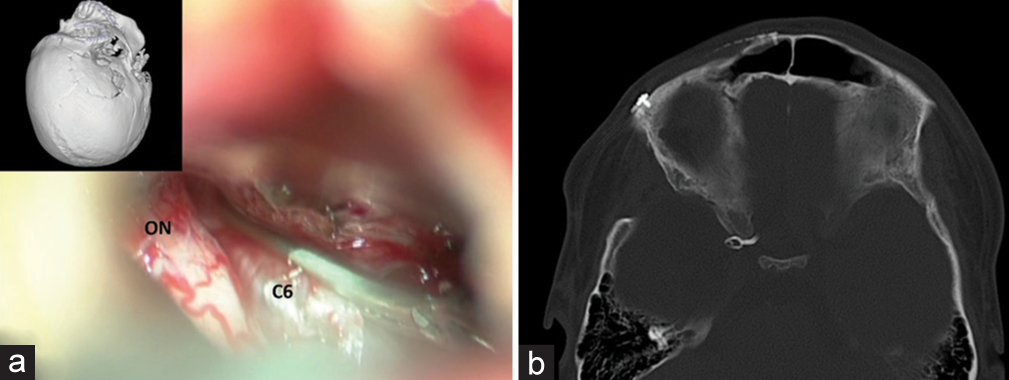
On the following day, the patient underwent the right frontotemporal craniotomy. The right optic nerve and the ophthalmic segment of the right ICA were identified under microscope [
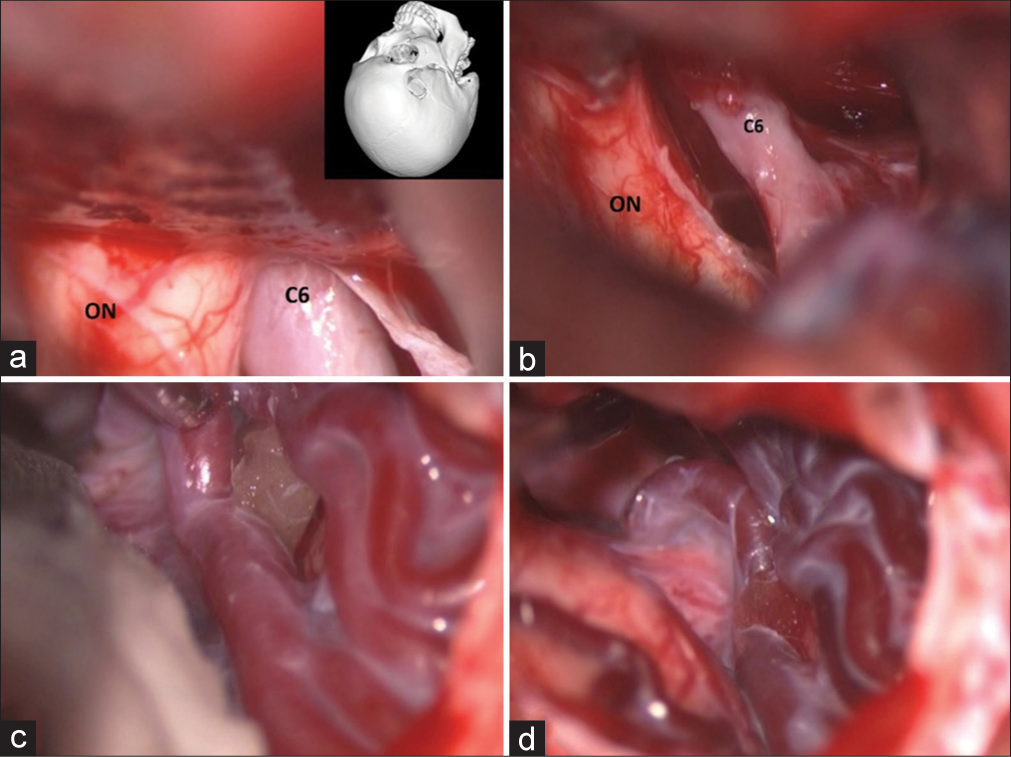
Figure 13:
Patient 2. Intraoperative photographs through the right pterional approach. (a, b) Identifying the right optic nerve (ON) and the ophthalmic segment of the right internal carotid artery (C6). After clipping the C6, illustrating the right arterialized Sylvian veins (c) before and (d) after placing the clip.
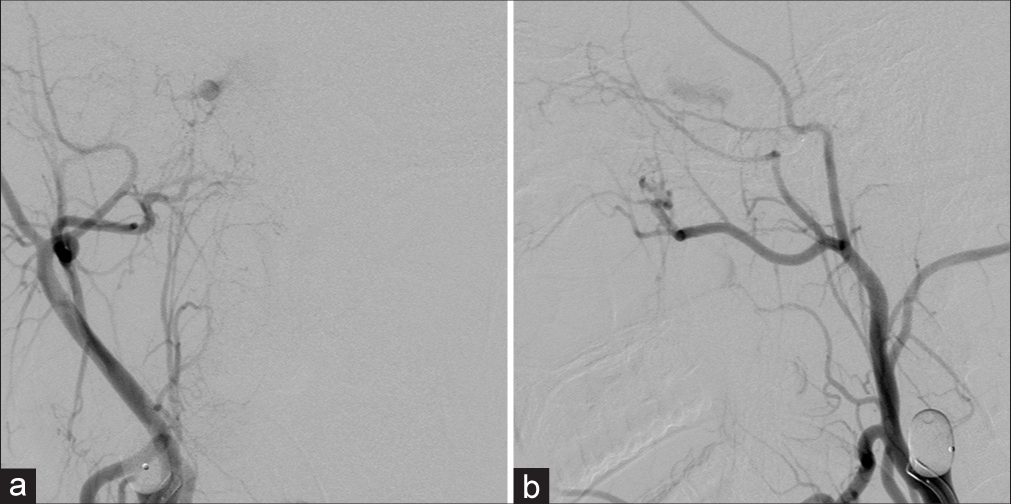
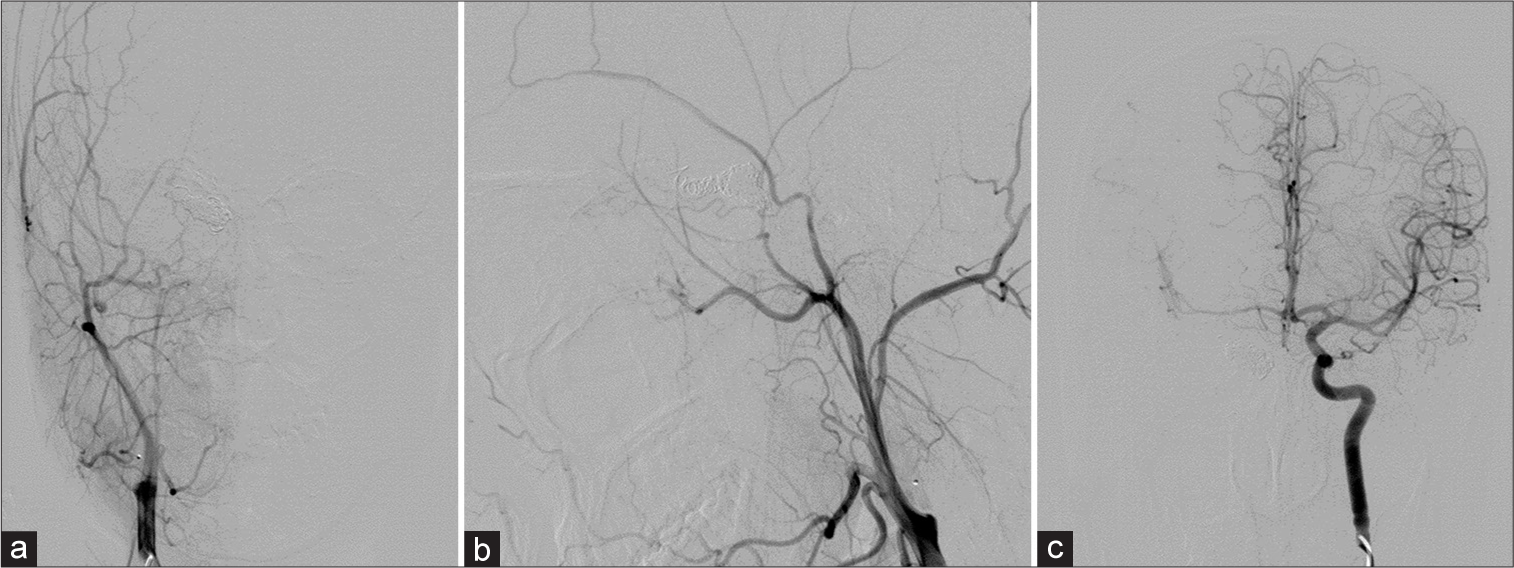
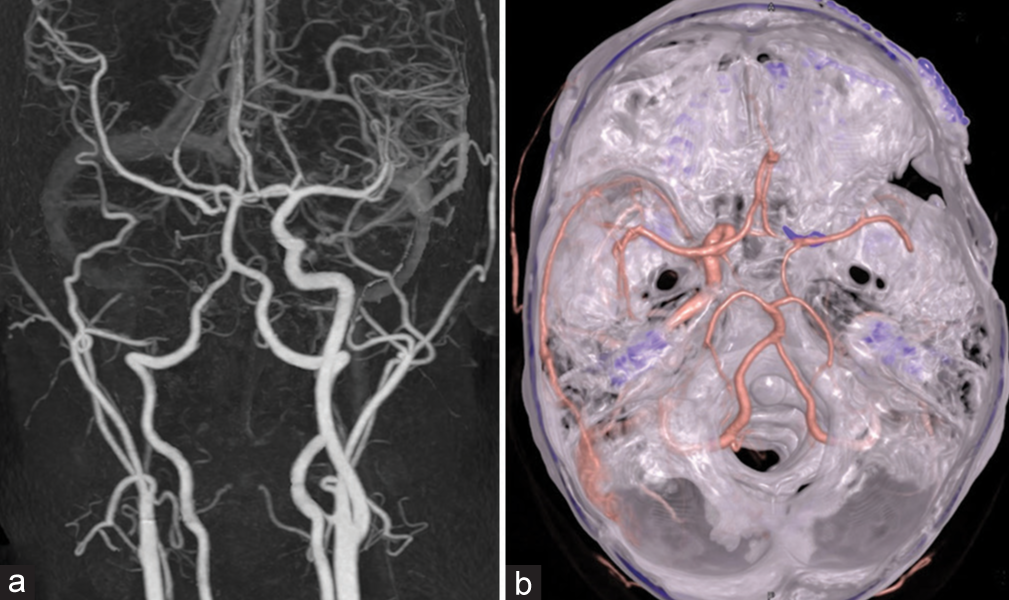
Follow-up cerebral angiography, obtained 2 weeks after surgery, revealed the small residual fistula supplied by the right OA reconstituted from the right middle meningeal artery and the clival branch from the left MHT with draining into the right SOV, IPS, cortical, and deep venous systems. The left vertebral angiography filled the right middle cerebral artery (MCA) through the right PCoA and the left ICA filled the right anterior cerebral artery (ACA) through ACoA. In addition, maximum intensity projection reformatted images of angiographic CT illustrated the disappearance of the pseudoaneurysm within the sphenoid sinus. Transvenous embolization was subsequently carried out through the right IPS using fibered interlocking detachable coils (Interlock-35, Boston Scientific, Natick, MA) densely packing in the proximal right SOV and within the CS [
Figure 14:
Patient 2. (a) Axial view of cranial computed tomography (CT) bone window scan demonstrates the surgical clip near the right anterior clinoid process. Sagittal maximum intensity projection reformatted images of angiographic CT (b) before and (c) after surgery reveal the disappearance of the pseudoaneurysm within the sphenoid sinus. Lateral views of (d) arterial and (e) venous phases of the right external carotid artery injection reveal the residual fistula fed by the right ophthalmic artery (asterisk) reconstituted from the right middle meningeal artery. (f) AP view of the left internal carotid artery injection demonstrates the clival branch (arrow) from the left meningohypophyseal trunk supplying the fistula. (g) AP and (h) lateral views of the left vertebral artery injection show good collateral supply of the right cerebral hemisphere from the vertebrobasilar system via the right posterior communicating artery. (i) Lateral view of unsubtracted image illustrates the entire coil mesh after embolization.
Case 3
A 16-year-old right-handed man was involved in a motorcycle accident and sustained a severe head injury. Cranial CT scan showed acute subdural hematoma at right frontotemporal region and multiple fractures affecting the right frontal bone, orbital bone, zygoma, skull base, ethmoid bone, and sphenoid bone with bone spicule within the sphenoid sinus [
Figure 16:
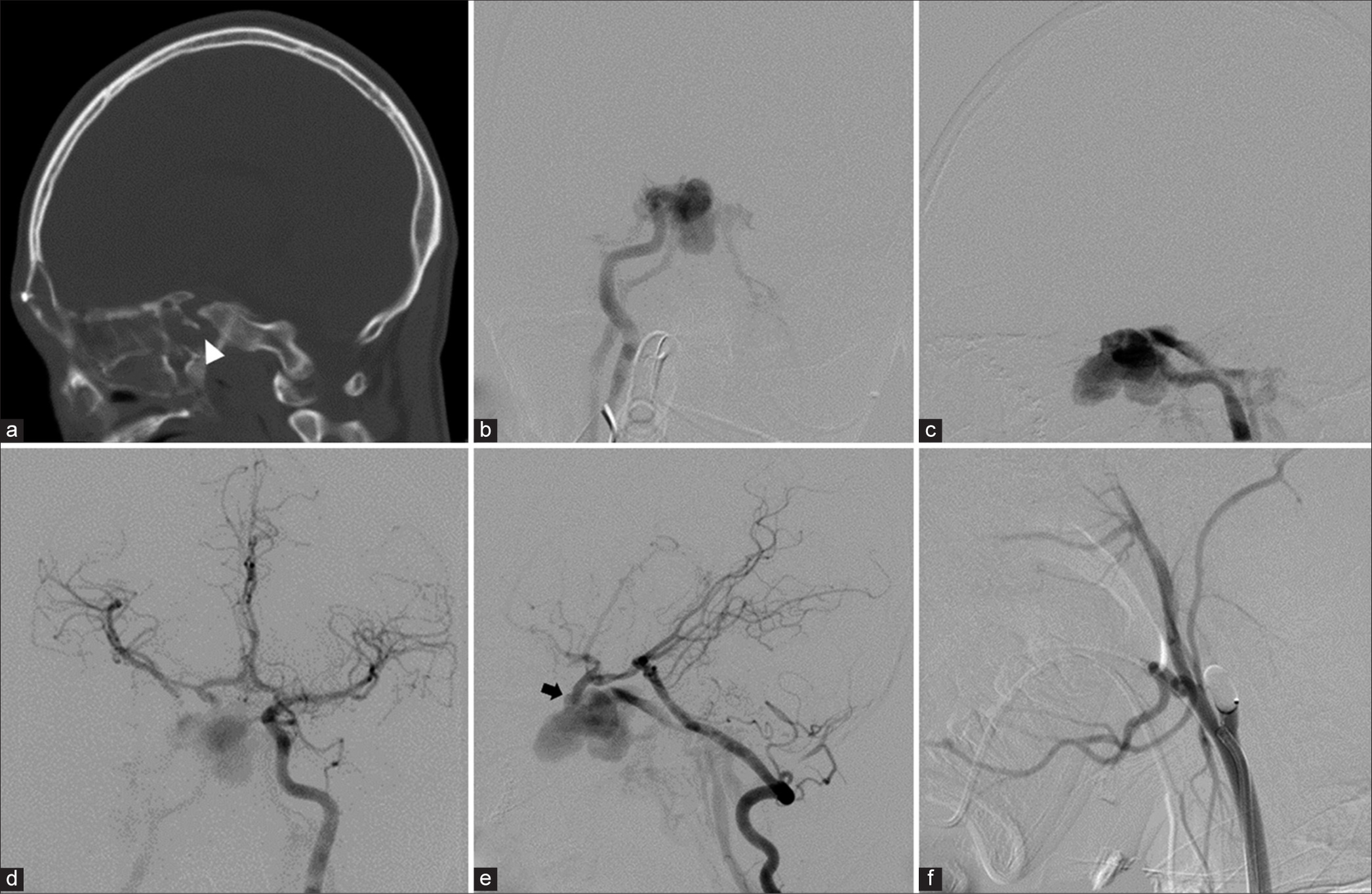
Patient 3. (a) Sagittal view of bone-window computed tomography scan show multiple skull base fractures with fractured bone spicule (arrowhead) within the sphenoid sinus. (b) Anteroposterior and (c) lateral views of the right internal carotid artery (ICA) injection reveals direct high-flow carotid-cavernous fistula with large lobulated pseudoaneurysm. (d) Anteroposterior view of the left ICA injection demonstrates collateral flow via the anterior communicating artery. (e) Lateral view of the left vertebral artery injection with compression of the right common carotid artery (CCA) shows the fistula (arrow) between clinoidal segment of the right ICA and cavernous sinus. (f) Lateral view of the right CCA confirms complete obliteration of the right ICA following the detachable balloon occlusion.
Cerebral angiography was carried out and disclosed direct high-flow fistula from medial tear of the clinoidal segment of the right ICA draining into dilated right CS with a large lobulated out-pouching pseudoaneurysm protruding through into the right sphenoid sinus. The pseudoaneurysm arising from medial aspect of the right clinoidal ICA and extending inferiorly into sphenoid sinus [
Under general anesthesia and heparinization, the first detachable balloon was successfully placed across the orifice of the fistula, and another balloon was placed at the cervical portion of the right ICA. The right CCA injection confirmed complete occlusion of the right carotid artery [
On the same day, the patient was moved to operating room and underwent the right frontotemporal craniotomy. The right optic nerve and the ophthalmic segment of the right ICA were identified under microscope [
Figure 17:
Patient 3. (a) Microscopic photograph after the right pterional craniotomy reveals the right optic nerve (ON) and the ophthalmic segment (C6) of the right internal carotid artery. (b) axial view of cranial computed tomography bone window scan demonstrates the surgical clip near the right anterior clinoid process.
Follow-up cerebral angiography, obtained 1 month after surgery, confirmed complete obliteration of the fistula and associated pseudoaneurysm [
Figure 18:
Patient 3. (a) Lateral view of the right common carotid artery, anteroposterior (AP) view of the left common carotid artery, and AP and lateral views of the left vertebral artery injections, obtained 1 month after treatment, confirm complete obliteration of the fistula and associated pseudoaneurysm.
DISCUSSION
The evolution of the various strategies used to treat TCCF
Over the past 200 years, the treatments of high-flow TCCF have been developed with four main principles including the obstruction of the flow in the arterial afferent to the fistula such as ligation of the carotid artery, the occlusion of the venous exits of the CS such as ligation of the SOV, the promotion of thrombosis formation within the CS, and direct closure at the fistula site.[
In 1809, the first treatment strategy for TCCF was the ligation of CCA.[
Based on the review of surgically treated cases of direct CCFs by Hamby,[
Before an era of advanced embolization technique, Hamby[
Since 1982, there were several reports of complications of detachable balloon catheter technique in the treatment of TCCFs such as inadvertent balloon detachment, the balloon rupture, early deflation, or shrinkage of the balloon, allowing the fistula to reopen, production of cranial nerve palsies when multiple balloons are used.[
Recurrent TCCF following muscle embolization, ICA ligation, or combination
The incidence of recurrent TCCF following the carotid artery ligation remains unknown. Patients harboring recurrent TCCF usually presented with ocular symptoms from dilated affected SOV.[
In the past three decades, the use of muscle embolization, trapping, and/or cervical carotid artery ligation remained to be utilized in our country due to extenuating circumstances such as poor socioeconomic status.[
In case of recurrent TCCF with previously ligated ICA, the fistula can be closed through different routes including transarterial through ACoA from the contralateral ICA or PCoA from the vertebrobasilar system, or transvenous route through SOV or IPS, direct surgical exposure of the CS, and direct puncture or exposure through the remnant of the affected cervical ICA above the occlusive site.[
In our first case, we first attempted embolization with detachable balloon through ACoA and PCoA, but it was difficult to navigate the balloon catheter into the fistula due to relatively stiff device. Therefore, we used the microcatheter navigating through ACoA from the contralateral ICA for embolization with coils. Unfortunately, the retrograde shunt into the fistula was so great that the coils mass was unable and kept migrating into the venous pouch. Then, we decided to trap this fistula with coiling proximal and distal to the fistula. However, it was impossible to safely pack the supraclinoid ICA after packing the petrous segment ICA. The fistula showed inaccessible through draining venous systems. Consequently, we chose intracranial clipping of the supraclinoid ICA.
Chronic recurrent or occult TCCF after trapping operation
The presence of a long-standing TCCF, which was sustained more than 20 years, is extremely rare.[
O’Reilly et al.[
The fistula may persist or recur if the cavernous segment of ICA is not completely thrombosed following the trapping procedure.[
TCCF in association with extensive venous congestive encephalopathy (EVCE)
In cerebral vascular lesions such as brain arteriovenous malformations and dural arteriovenous fistulas, EVCE is condition when too much blood drained into the venous systems involving extensive cerebral veins. The progression of EVCE can be divided into acute and chronic stages. This condition often indicates a more aggressive natural history. The common clinical manifestations, the consequence of venous congestion, usually consist of headache, cognitive impairment, seizure, focal deficits, and/or hemorrhage. Progressive cognitive impairment is often in chronic stage. At chronic stage, tortuous, dilated, and engorged veins with calcification could be seen on imaging findings. Prompt treatment is warranted, and complete elimination is often difficult. Therefore, staged treatment may be chosen. Aggressive treatment is effective and results in an acceptable prognosis.[
Concomitant TCCF and pseudoaneurysm within the sphenoid sinus
Patients harboring the traumatic pseudoaneurysm within the sphenoid sinus usually exhibit the classic triad of unilateral blindness, orbital fracture, and massive epistaxis.[
The management of a TCCF associated with traumatic aneurysm in sphenoid sinus can be challenging.[
Recently, Cho et al.[
Trapping procedure by combined endovascular and surgical approach for TCCF
The management of CCF is variable and depends on the anatomy of the fistula. Surgical assistance for TCCF should be limited to those case when the affected ICA cannot be preserved, or when the affected ICA has been ligated previously.[
Combined endovascular and surgical treatments for concurrent of a TCCF and sphenoid sinus pseudoaneurysm have rarely been reported. At present, Ghorbani et al.[
Another technique of endovascular trapping of TCCF by Coley et al.,[
For sacrifice the affected ICA with balloon, the balloon should be inflated against the opening of the fistula.[
Follow-up cerebral angiography is essential to verify a cure of TCCF after the trapping procedure. Following trapping procedure for TCCF, the recurrent or residual fistula can be reached through different approaches including direct surgical approach, direct percutaneous transorbital approach, or transvenous approach through SOV or IPS. [
Teng et al.[
The development of indirect CCF following the treatment of TCCF
Yoshino et al.[
The venous approach may be indicated when the fistula has been previously trapped but is still function.[
CONCLUSION
The treatment of TCCF has evolved from surgery to endovascular management using detachable balloon or coils through arterial or venous approach. The endovascular method currently is the treatment of choice for TCCF due to its ability to preserve the carotid artery and flexibility in treatment strategy with various approaches to the fistula. However, surgical assistance may remain useful for some atypical TCCFs.
To reduce costs of treatment, trapping operation by combining surgical and endovascular treatment may be considered as an alternative option for complex TCCF which has some characteristics including chronic stage, preexisting carotid artery ligation, or association with large venous pouch of the cavernous sinus or sphenoid sinus pseudoaneurysm.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barrow DL, Fleischer AS, Hoffman JC. Complications of detachable balloon catheter technique in the treatment of traumatic intracranial arteriovenous fistulas. J Neurosurg. 1982. 56: 396-403
2. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985. 62: 248-56
3. Browder J. Treatment of carotid artery-cavernous sinus fistula: Report of a case. Arch Ophthalmol. 1937. 18: 95-102
4. Chen P, Dunn IF, Aglio LS, Day AL, Frerichs KU, Friedlander RM. Intraoperative awakening for vision examination during ophthalmic artery aneurysm clipping: Technical case report. Neurosurgery. 2005. 56: E440
5. Cho MJ, Yi KS, Choi CH, Yum KS, Cha SH, Kim Y. Sphenoid sinus pseudoaneurysm with carotid cavernous fistula presenting with delayed subarachnoid hemorrhage: A case report. Medicine (Baltimore). 2021. 100: e26383
6. Coley SC, Pandya H, Hodgson TJ, Jeffree MA, Deasy NP. Endovascular trapping of traumatic carotid-cavernous fistulae. AJNR Am J Neuroradiol. 2003. 24: 1785-8
7. Dandy WE. The treatment of carotid cavernous arteriovenous aneurysms. Ann Surg. 1935. 102: 916-26
8. Dang NV, Hai NT, Cuong TC. Recurrent carotico-cavernous fistula after internal carotid artery ligation: A case with embolization of the fistula via contralateral internal carotid artery approach. Interv Neuroradiol. 2014. 20: 482-6
9. Das S, Gupta AK, Ramalingiah AH, Tiwari S, Yadav N. Delayed migration of Squid 18 following embolisation of a direct carotico-cavernous fistula. Interv Neuroradiol. 2018. 24: 210-3
10. Debrun G, Legre J, Kasbarian M, Tapias PL, Caron JP. Endovascular occlusion of vertebral fistulae by detachable balloons with conservation of the vertebral blood flow. Radiology. 1979. 130: 141-7
11. Debrun GM, Ausman JI, Charbel FT, Aletich VA. Access to the cavernous sinus through the vertebral artery: Technical case report. Neurosurgery. 1995. 37: 144-6
12. Debrun GM, Nauta HJ, Miller NR, Drake CG, Heros RC, Ahn HS. Combining the detachable balloon technique and surgery in imaging carotid cavernous fistulae. Surg Neurol. 1989. 32: 3-10
13. Debrun GM, Viñuela F, Fox AJ, Davis KR, Ahn HS. Indications for treatment and classification of 132 carotid-cavernous fistulas. Neurosurgery. 1988. 22: 285-9
14. Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983. 58: 824-31
15. Ducruet AF, Albuquerque FC, Crowley RW, McDougall CG. The evolution of endovascular treatment of carotid cavernous fistulas: A single-center experience. World Neurosurg. 2013. 80: 538-48
16. Echols DH, Jackson JD. Carotid-cavernous fistula: A perplexing surgical problem. J Neurosurg. 1959. 16: 619-27
17. Fox AJ, Viñuela F, Pelz DM, Peerless SJ, Ferguson GG, Drake CG. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg. 1987. 66: 40-6
18. Garcia-Cervigon E, Bien S, Laurent A, Weitzner I, Biondi A, Merland JJ. Treatment of a recurrent traumatic carotid-cavernous fistula: Vertebro-basilar approach after surgical occlusion of the internal carotid artery. Neuroradiology. 1988. 30: 355-7
19. Ghorbani M, Griessenauer CJ, Wipplinger C, Abdolhoseinpour H, Bahrami R, Asaadi S. Combined endovascular and endoscopic approach for treatment of concomitant sphenoid sinus giant traumatic aneurysm and direct carotid cavernous fistulas. World Neurosurg. 2020. 134: 211-4
20. Gurdjian S. Packing of internal carotid artery with muscle in treatment of carotid-cavernous arteriovenous aneurysm. Arch Ophthalmol. 1938. 19: 936-40
21. Halbach VV, Higashida RT, Hieshima GB, Hardin CW. Direct puncture of the proximally occluded internal carotid artery for treatment of carotid cavernous fistulas. AJNR Am J Neuroradiol. 1989. 10: 151-4
22. Hamby WB. Carotid-cavernous fistula, report of 32 surgically treated cases and suggestions for definitive operation. J Neurosurg. 1964. 21: 859-66
23. Hou K, Song Y, Guo Y, Yu J. Brain arteriovenous malformations and dural arteriovenous fistulas with extensive venous congestive encephalopathy. Acta Neurol Belg. 2022. 122: 1-9
24. Huai RC, Yi CL, Ru LB, Chen GH, Guo HH, Luo L. Traumatic carotid cavernous fistula concomitant with pseudoaneurysm in the sphenoid sinus. Interv Neuroradiol. 2008. 14: 59-68
25. Iampreechakul P, Tanpun A, Lertbusayanukul P, Siriwimonmas S. Contralateral extensive cerebral hemorrhagic venous infarction caused by retrograde venous reflux into the opposite basal vein of Rosenthal in posttraumatic carotid-cavernous fistula: A case report and literature review. Interv Neuroradiol. 2018. 24: 546-58
26. Iampreechakul P, Tirakotai W, Tanpun A, Wattanasen Y, Lertbusayanukul P, Siriwimonmas S. Spontaneous resolution of direct carotid-cavernous fistulas: Case series and literature review. Interv Neuroradiol. 2019. 25: 71-89
27. Iampreechakul P, Wangtanaphat K, Hangsapruek S, Wattanasen Y, Lertbutsayanukul P, Siriwimonmas S. Transfemoral transvenous embolization through the vein of Trolard and superficial middle cerebral vein for cavernous sinus dural arteriovenous fistula with isolated cortical vein drainage: A case report and literature review. Surg Neurol Int. 2022. 13: 34
28. Isamat F, Ferrer E, Twose J. Direct intracavernous obliteration of high-flow carotid-cavernous fistulas. J Neurosurg. 1986. 65: 770-5
29. Kapp JP, Pattisapu JR, Parker JL. Transarterial closure of persistent carotid-cavernous fistula after carotid ligation. Case report. J Neurosurg. 1984. 61: 402-4
30. Koekkoek JA, Lycklama à Nijeholt GJ, Jellema K, Walchenbach R. Traumatic carotid-cavernous fistula combined with pseudoaneurysm requires immediate treatment. BMJ Case Rep. 2013. 2013: bcr2013009013
31. Komiyama M, Yasui T, Yagura H, Fu Y, Nagata Y. Traumatic carotid-cavernous sinus fistula associated with an intradural pseudoaneurysm: A case report. Surg Neurol. 1991. 36: 126-32
32. Lang ER, Bucy PC. Treatment of carotid-cavernous fistula by muscle embolization alone: The brooks method. J Neurosurg. 1965. 22: 387-92
33. Langford KH, Vitek JJ, Zeiger E. Migration of detachable mini-balloon from the ICA causing occlusion of the MCA. Case report. J Neurosurg. 1983. 58: 430-4
34. Lewis AI, Tomsick TA, Tew JM. Management of 100 consecutive direct carotid-cavernous fistulas: Results of treatment with detachable balloons. Neurosurgery. 1995. 36: 239-44
35. Lin TC, Mao SH, Chen CH, Chen YL, Wong HF, Chang CJ. Systematic analysis of the risk factors affecting the recurrence of traumatic carotid-cavernous sinus fistula. World Neurosurg. 2016. 90: 539-45.e1
36. Locke CE. Intracranial arterio-venous aneurism or pulsating exophthalmos. Ann Surg. 1924. 80: 1-24
37. Lv XL, Li YX, Liu AH, Lv M, Jiang P, Zhang JB. A complex cavernous sinus dural arteriovenous fistula secondary to covered stent placement for a traumatic carotid artery-cavernous sinus fistula: Case report. J Neurosurg. 2008. 108: 588-90
38. Maurer JJ, Mills M, German WJ. Triad of unilateral blindness, orbital fractures and massive epistaxis after head injury. J Neurosurg. 1961. 18: 837-40
39. Mullan S. Treatment of carotid-cavernous fistulas by cavernous sinus occlusion. J Neurosurg. 1979. 50: 131-44
40. Norland L, Taylor AS. Pulsating exophthalmos, result of injury. Trans South Surg Ass. 1931. 43: 171-7
41. O’Reilly GV, Shillito J, Haykal HA, Kleefield J, Wang AM, Rumbaugh CL. Balloon occlusion of a recurrent carotid-cavernous fistula previously treated by carotid ligations. Neurosurgery. 1986. 19: 643-8
42. Palestine AG, Younge BR, Piepgras DG. Visual prognosis in carotid-cavernous fistula. Arch Ophthalmol. 1981. 99: 1600-3
43. Parkinson D. A surgical approach to the cavernous portion of the carotid artery. Anatomical studies and case report. J Neurosurg. 1965. 23: 474-83
44. Sanders MD, Hoyt WF. Hypoxic ocular sequelae of carotid-cavernous fistulae. Study of the caues of visual failure before and after neurosurgical treatment in a series of 25 cases. Br J Ophthalmol. 1969. 53: 82-97
45. Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974. 41: 125-45
46. Stern WE, Brown WJ, Alksne JF. The surgical challenge of carotid-cavernous fistula: The critical role of intracranial circulatory dynamics. J Neurosurg. 1967. 27: 298-308
47. Teng MM, Guo WY, Lee LS, Chang T. Direct puncture of the cavernous sinus for obliteration of a recurrent carotid-cavernous fistula. Neurosurgery. 1988. 23: 104-7
48. Terada T, Miyatake N, Naka D, Tsuura M, Matsumoto H, Masuo O. Indirect carotid cavernous fistula appeared after balloon embolization of direct CCF. Acta Neurochir (Wien). 2002. 144: 489-92
49. Thanapura C. Treatment of traumatic carotid-cavernous fistula at the Udon Thani Center Hospital. J Clin Neurosci. 2004. 11: 498-500
50. Travers B. A case of aneurysm by anastomosis in the orbit, cured by the ligature of the common carotid artery. Trans Med Chir Soc. 1809. 2: 1-16
51. Van Dellen JR. Intracavernous traumatic aneurysms. Surg Neurol. 1980. 13: 203-7
52. van Rooij WJ, Sluzewski M, Beute GN. Ruptured cavernous sinus aneurysms causing carotid cavernous fistula: Incidence, clinical presentation, treatment, and outcome. AJNR Am J Neuroradiol. 2006. 27: 185-9
53. Workman MJ, Dion JE, Tong FC, Cloft HJ. Treatment of trapped CCF by direct puncture of the cavernous sinus by infraocular trans-SOF approach. Case report and anatomical basis. Interv Neuroradiol. 2002. 8: 299-304
54. Yoshino H, Ishihara H, Oka F, Kato S, Suzuki M. Development of indirect cavernous dural arteriovenous fistula after trapping for direct carotid cavernous fistula. A case report. Interv Neuroradiol. 2011. 17: 104-7