- Department of Neurosurgery, Nara City Hospital, Nara, Japan
- Department of Pathology, Nara City Hospital, Nara, Japan
Correspondence Address:
Takayuki Morimoto, Department of Neurosurgery, Nara City Hospital, Nara, Japan.
DOI:10.25259/SNI_487_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takayuki Morimoto1, Sung-Chul Ko1, Keiji Shimada2, Toshikazu Nishioka1, Hidemori Tokunaga1. Combined endovascular therapy and surgery for central giant cell granuloma in the temporal bone: A case report. 20-Sep-2024;15:335
How to cite this URL: Takayuki Morimoto1, Sung-Chul Ko1, Keiji Shimada2, Toshikazu Nishioka1, Hidemori Tokunaga1. Combined endovascular therapy and surgery for central giant cell granuloma in the temporal bone: A case report. 20-Sep-2024;15:335. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13111
Abstract
Background: Central giant cell granuloma (CGCG) is an uncommon, benign intraosseous lesion that most frequently occurs in the mandible and maxilla.
Case Description: A 31-year-old female with a medical history of Kawasaki disease presented to our hospital complaining of a clogged right ear. Head computed tomography revealed a mass in the squamous part of the right temporal bone, with osteolytic changes and invasion of the external auditory canal, middle ear, temporomandibular joint, and mastoid air cells. Enhanced magnetic resonance imaging (MRI) showed a strong signal in the intraosseous lesion. Digital subtraction angiography revealed tumor staining from multiple feeders, including the middle meningeal, posterior deep temporal, and posterior auricular arteries. Preoperative feeder embolization using a detachable coil and Embosphere Microspheres were performed for the middle meningeal artery under general anesthesia. After the endovascular treatment, we operated on the temporal bone lesion. Postoperative enhanced MRI showed subtotal resection and residual tumor near the external auditory canal, which was left in place to prevent opening the external auditory canal. The histopathological examination showed proliferation of mononuclear cells intermingled with osteoclast-like multinucleated giant cells. A diagnosis of CGCG was made. The postoperative course was uncomplicated, and the patient was discharged on day 10 of hospitalization.
Conclusion: We reported a rare case of CGCG in the temporal bone, managed by endovascular therapy and surgical resection. This combination therapy resulted in subtotal resection, preserving surrounding normal structures, such as the external auditory canal and tympanic cavity.
Keywords: Central giant cell granuloma, Feeder embolization, Temporal bone
INTRODUCTION
Central giant cell granulomas (CGCGs) are rare benign lesions originating from bone tissue and occurring mostly in the mandible and maxilla.[
CASE REPORT
A 31-year-old female patient with a history of fully-healed Kawasaki disease and no head trauma presented to our outpatient Department of Otolaryngology complaining of a clogged right ear. Head computed tomography (CT) revealed an osteolytic lesion in the right temporal bone that extended to the external auditory canal (EAC), middle ear, the roof of the temporomandibular joint (TMJ), and mastoid air cells [
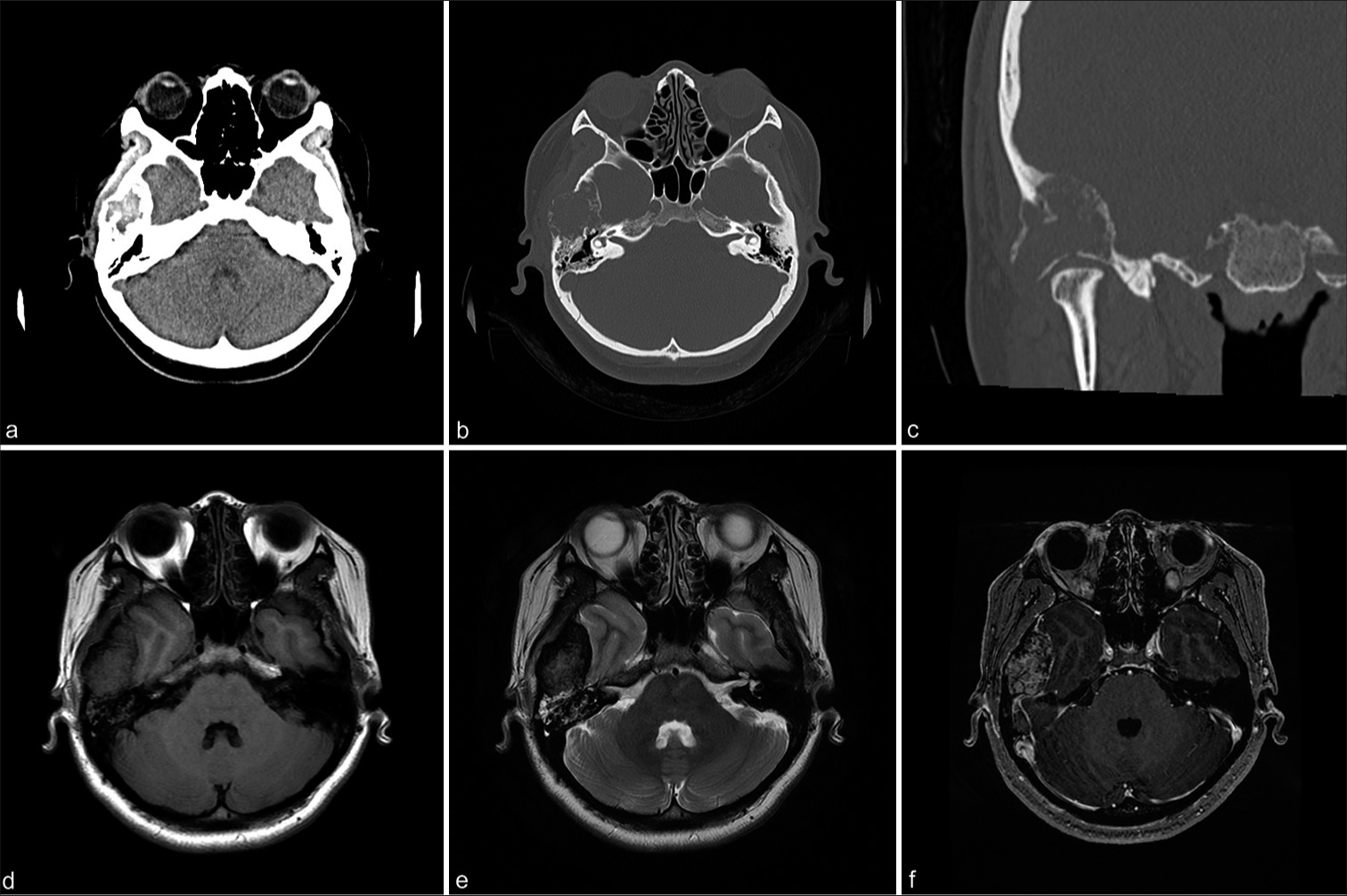
Figure 1:
(a) Computed tomography (CT) demonstrating a temporal bone tumor. (b, c) CT bone window shows an expansive and destructive process in the temporal bone that extends to the external auditory canal, middle ear, temporomandibular joint roof, and mastoid air cells. (d, e) Initial head magnetic resonance imaging (MRI) revealed a well-delineated solid extra-axial isointense signal in the right temporal bone in T1 and T2 sequences. (f) A gadolinium contrast MRI T1 sequence shows strong enhancement.
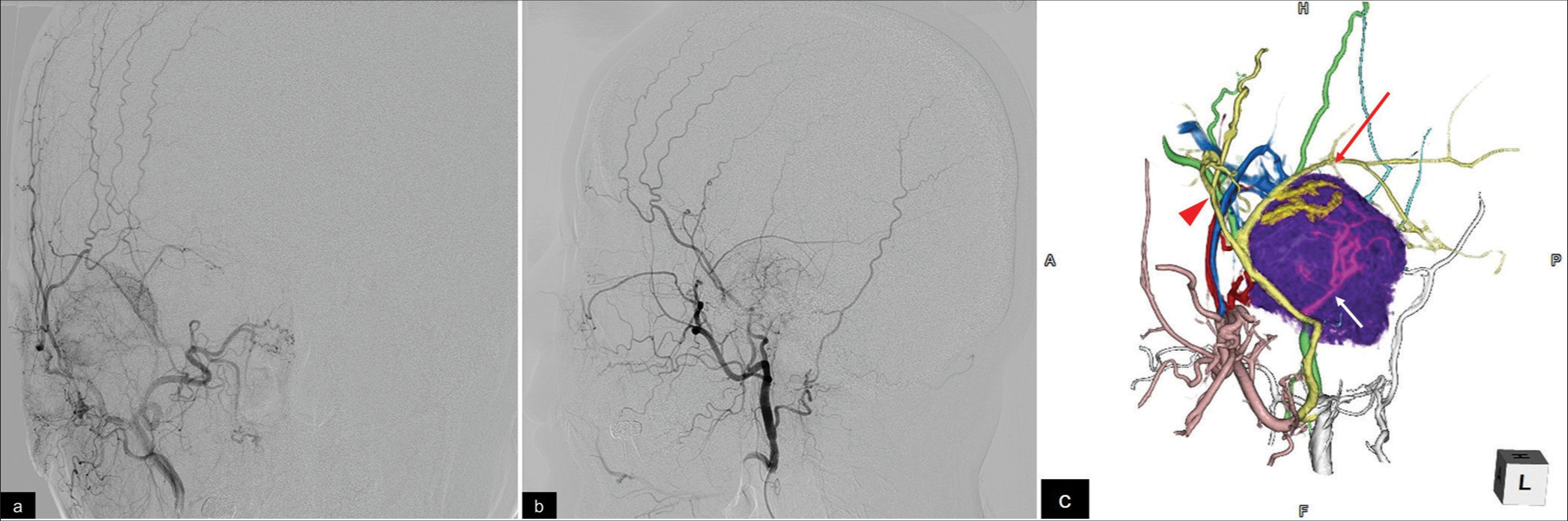
Figure 2:
(a, b)Right external carotid artery angiography reveals multiple feeders for the bone tumor, including the posterior convexity and petrosquamous branches of the middle meningeal artery, posterior deep temporal artery, and posterior auricular artery (a, anterior view; b, lateral view). (c) Three-dimensional volume rendering shows tumor (purple) and frontal (red arrow head), petrosquamous (red arrow), and petrosal (white arrow) branch of MMA.
Under general anesthesia, a 6 Fr FUBUKI catheter (Asahi Intecc, Nagoya, Aichi, Japan) was navigated to the right ECA. An intermediate catheter (Guidepost; Tokai Medical Products, Aichi, Japan) was then advanced into the orifice of the MMA. An Excelsior 1018 microcatheter (Stryker, Kalamazoo, MI, USA) was inserted into the posterior convexity branch of MMA using a microwire (SynchroSELECT standard; Stryker); [
A CGCG was suspected based on the pathology report of an intraoperative frozen sample. Hematoxylin-eosin staining of the resected lesion showed multinucleated osteoclast-like giant cells with spindle-shaped or polygonal stromal cells [
Figure 3:
(a) Microcatheter was introduced into the posterior convexity branch of the middle meningeal artery (MMA), and an Embosphere microsphere (300–500 µm) was injected. (b) Embolization for the main MMA trunk, using a detachable coil. (c) External carotid artery angiography shows that the stain disappeared from the MMA. (d) Operative images show a firm, partially bony, reddish-brown tumor. (e) Hematoxylin-eosin staining shows multinucleated osteoclast-like giant cells with spindle-shaped or polygonal stromal cells. (f) Prussian blue staining shows the presence of hemosiderin. (g, h) Postoperative enhanced magnetic resonance imaging shows subtotal resection of the temporal bone tumor.
DISCUSSION
CGCGs were first reported by Jaffe[
The pathogenesis of CGCG is unclear. It is assumed to be a hyperplastic reparative and self-healing reaction to intra-osseous trauma-induced hemorrhage that triggers the reactive granulomatous process.[
CGCG and GCT differ in their clinical course. Higher recurrence rates and risk of metastasis and malignant transformation were reported in GCT.[
The in-operative bleeding tendency of CGCG makes it a risk factor that hinders complete resection.[
CONCLUSION
We reported a rare case of CGCG in the temporal bone. The patient presented with a complaint of a clogged right ear. Combining endovascular therapy and surgical resection resulted in subtotal resection while preserving the surrounding normal vital structures, such as the external auditory canal and tympanic cavity.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, Van Loo P. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013. 45: 1479-82
2. Bernard F, Troude L, Bouvier C, Roche PH. Giant cell reparative tumor: An exceptional differential diagnosis for a lytic lesion of the temporal bone. Neurochirurgie. 2016. 62: 332-5
3. Boedeker CC, Kayser G, Ridder GJ, Maier W, Schipper J. Giant-cell reparative granuloma of the temporal bone: A case report and review of the literature. Ear Nose Throat J. 2003. 82: 926-9 933-4, 936-7
4. Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: A clinicopathologic study. J Oral Maxillofac Surg. 1986. 44: 708-13
5. Dai WY, Tian C, Liu L. Case reports of a giant cell reparative granuloma and a giant cell tumor on temporal bone. Chin Med J (Engl). 2018. 131: 2254-6
6. Dimitrakopoulos I, Lazaridis N, Sakellariou P, Asimaki A. Giant-cell granuloma in the temporal bone: A case report and review of the literature. J Oral Maxillofac Surg. 2006. 64: 531-6
7. Jaffe HL. Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol. 1953. 6: 159-75
8. Lewis ML, Weber AL, McKenna MJ. Reparative cell granuloma of the temporal bone. Ann Otol Rhinol Laryngol. 1994. 103: 826-8
9. Li X, Wen Y, Zhang J, Wu N, Shen W, Yang S. Imaging features, staging system, and surgical management of giant cell lesions of the temporal bone. Acta Otolaryngol. 2022. 142: 553-61
10. Matsui T, Iwamuro K, Ishikawa T, Asano T, Itoyama S, Tabe H. Large giant cell reparative granuloma of the petrous bone--case report. Neurol Med Chir (Tokyo). 2002. 42: 232-6
11. Montero EH, Navarro JS, Pueyo JL, Garca FM, Sampériz LC, Garca AO. Giant-cell reparative granuloma in the temporal bone. Am J Otolaryngol. 2003. 24: 191-3
12. Moser A, Hoffmann KM, Walch C, Sovinz P, Lackner H, Schwinger W. Intracranial reparative giant cell granuloma secondary to cholesteatoma in a 15-year-old girl. J Pediatr Hematol Oncol. 2008. 30: 935-7
13. Nemoto Y, Inoue Y, Tashiro T, Mochizuki T, Katsuyama J, Hakuba A. Central giant cell granuloma of the temporal bone. AJNR Am J Neuroradiol. 1995. 16: 982-5
14. Orosz Z, Athanasou NA. Giant cell-containing tumors of bone. Surg Pathol Clin. 2017. 10: 553-73
15. Sepulveda I, Alzerreca J, Villalobos P, Ulloa JP. Giant cell tumor of the temporal bone with skull base and middle ear extension. J Med Cases. 2021. 12: 149-51
16. Shrestha S, Zhang J, Yan J, Zeng X, Peng X, He B. Radiological features of central giant cell granuloma: Comparative study of 7 cases and literature review. Dentomaxillofac Radiol. 2021. 50: 20200429
17. Souter MA, Bird PA, Worthington JP. Giant cell reparative granuloma of the temporal bone treated with calcitonin. Otol Neurotol. 2006. 27: 999-1002
18. Thompson L. World Health Organization classification of tumours: Pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006. 85: 74