- Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan.
Correspondence Address:
Masahiro Sugihara, Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan.
DOI:10.25259/SNI_487_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahiro Sugihara, Atsushi Fujita, Yusuke Ikeuchi, Tatsuo Hori, Masaaki Kohta, Kazuhiro Tanaka, Hidehito Kimura, Takashi Sasayama. Combined transarterial and transvenous embolization of anterior cranial fossa dural arteriovenous fistula. 04-Aug-2023;14:277
How to cite this URL: Masahiro Sugihara, Atsushi Fujita, Yusuke Ikeuchi, Tatsuo Hori, Masaaki Kohta, Kazuhiro Tanaka, Hidehito Kimura, Takashi Sasayama. Combined transarterial and transvenous embolization of anterior cranial fossa dural arteriovenous fistula. 04-Aug-2023;14:277. Available from: https://surgicalneurologyint.com/surgicalint-articles/combined-transarterial-and-transvenous-embolization-of-anterior-cranial-fossa-dural-arteriovenous-fistula/
Abstract
Background: Excessive glue injection into the drainage vein in patients with dural arteriovenous fistula (dAVF) can result in venous obstruction. We performed transarterial embolization (TAE) combined with transvenous embolization (TVE) with coils to prevent the glue from migrating into the normal cortical veins.
Case Description: A 57-year-old man was pointed out to have a Borden Type III anterior cranial fossa dAVF during a check-up for putaminal hemorrhage. Because a left frontal normal cortical vein drained into the pathological drainage vein, excessive glue injection into the drainage vein may have caused venous obstruction. We performed TVE with coils at the foot of the draining vein to prevent excessive migration of glue into the drainer, followed by TAE with glue. With this technique, complete obliteration of the shunt without venous ischemia was obtained.
Conclusion: The combined treatment of TAE and TVE is effective in preventing venous ischemia caused by unintended migration of glue cast into the drainage vein.
Keywords: Anterior cranial fossa, Combined treatment, Dural arteriovenous fistula, Transarterial embolization, Transvenous embolization
INTRODUCTION
Anterior cranial fossa dural arteriovenous fistula (ACF dAVF) is a rare condition that accounts for approximately 10% of all dAVF cases. Retrograde drainage of the cortical veins is the most common drainage pattern and is characterized by a malignant course that most often presents with hemorrhage, which is reported to occur in 60–90% of cases.[
Accordingly, TAE carries the unavoidable risk of glue migration into the distal part of the draining vein until the shunt is completely filled with glue. In particular, TAE by Onyx often creates a relatively long cast into the draining vein; this cast may obstruct the orifice of normal cortical veins.
We report a patient with ACF dAVF successfully treated by glue embolization combined with TVE using coils. The affected drainage vein and the normal cortical veins in the left frontal lobe merged to form a common trunk and finally drained into the superior sagittal sinus (SSS). To avoid venous outflow impairment, the amount of glue injected into the drainage vein should be minimized. We performed TAE combined with TVE to prevent the migration of glue into the normal cortical vein. We controlled the blood flow by prefilling the foot of the drainage vein with a coil to prevent fragmentation of the glue. To the best of our knowledge, this is the first case report of a combined TAE and TVE technique for ACF dAVF. We addressed the safety and efficacy of our concept and discussed the technical aspects.
CASE REPORT
A 57-year-old man presented with a sudden onset of mild right upper and lower extremity paralysis. A magnetic resonance imaging (MRI) scan revealed a left capsular hemorrhage and scattered infarctions in the right middle cerebral artery and the patient was treated conservatively. Magnetic resonance angiography incidentally revealed findings suggestive of ACF dAVF, which was confirmed by cerebral angiography [
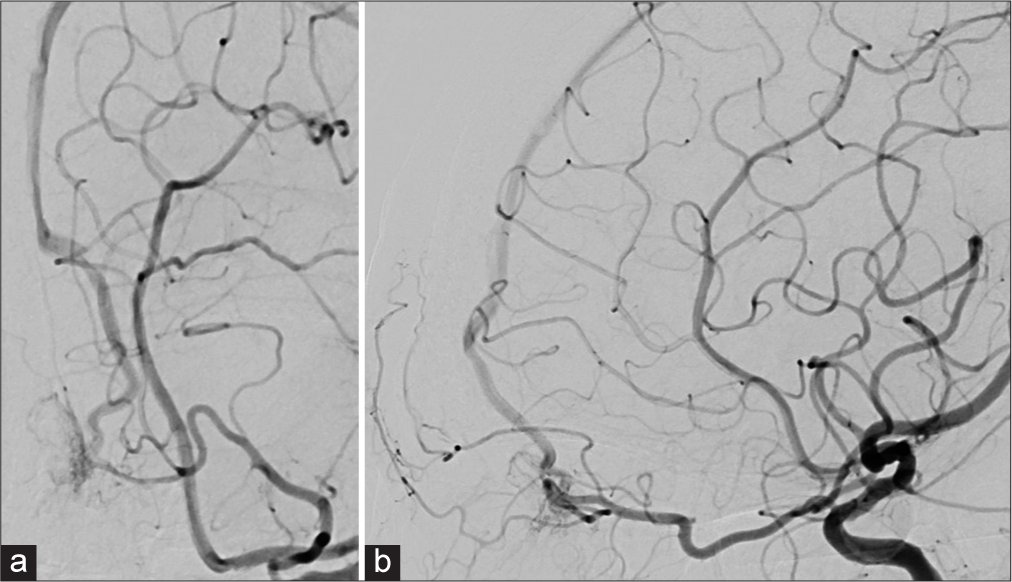
Figure 1:
(a) Left oblique and (b) lateral projections of the left internal carotid artery angiogram show the dural arteriovenous fistula at the anterior cranial fossa feeding by the left ophthalmic artery and venous reflux in the left frontal cortical veins drained into the anterior third of the superior sagittal sinus.
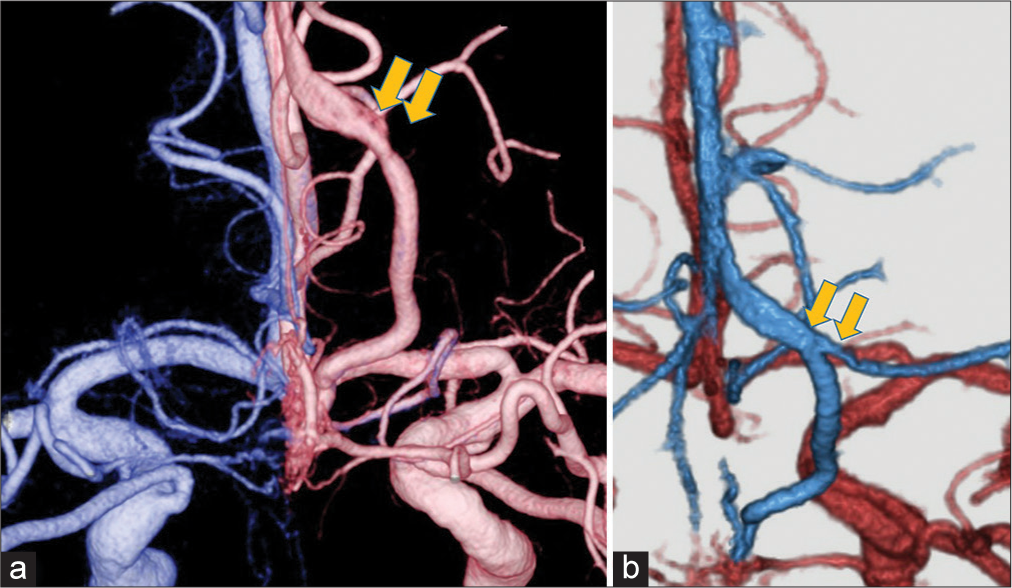
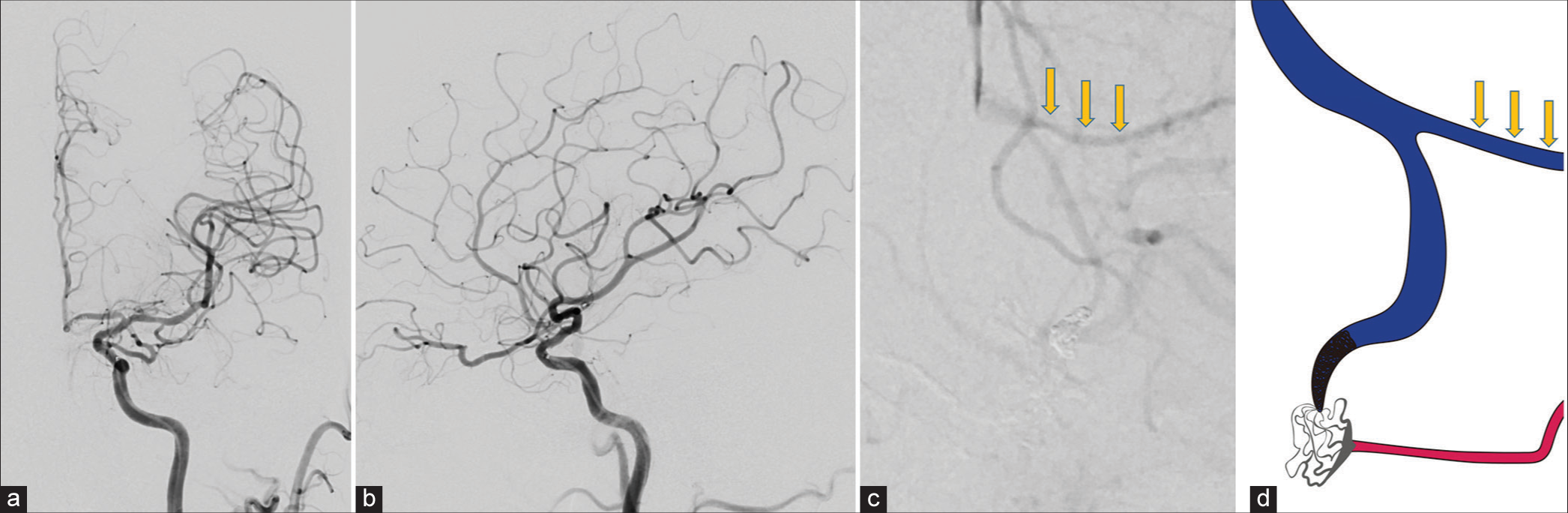
Figure 2:
(a) A fusion image of a three-dimensional bilateral internal carotid angiogram shows the anterior cranial fossa dural arteriovenous fistula with a drainage vein associated with a laminar flow (yellow arrow). (b) Computed tomography angiography reveals that it is the confluence of the affected drainage vein and the normal frontal cortical vein (yellow arrow). Both veins merge and form a common trunk that drains into the superior sagittal sinus.
The endovascular procedures were performed under general anesthesia using transfemoral artery and transinternal jugular vein approaches [
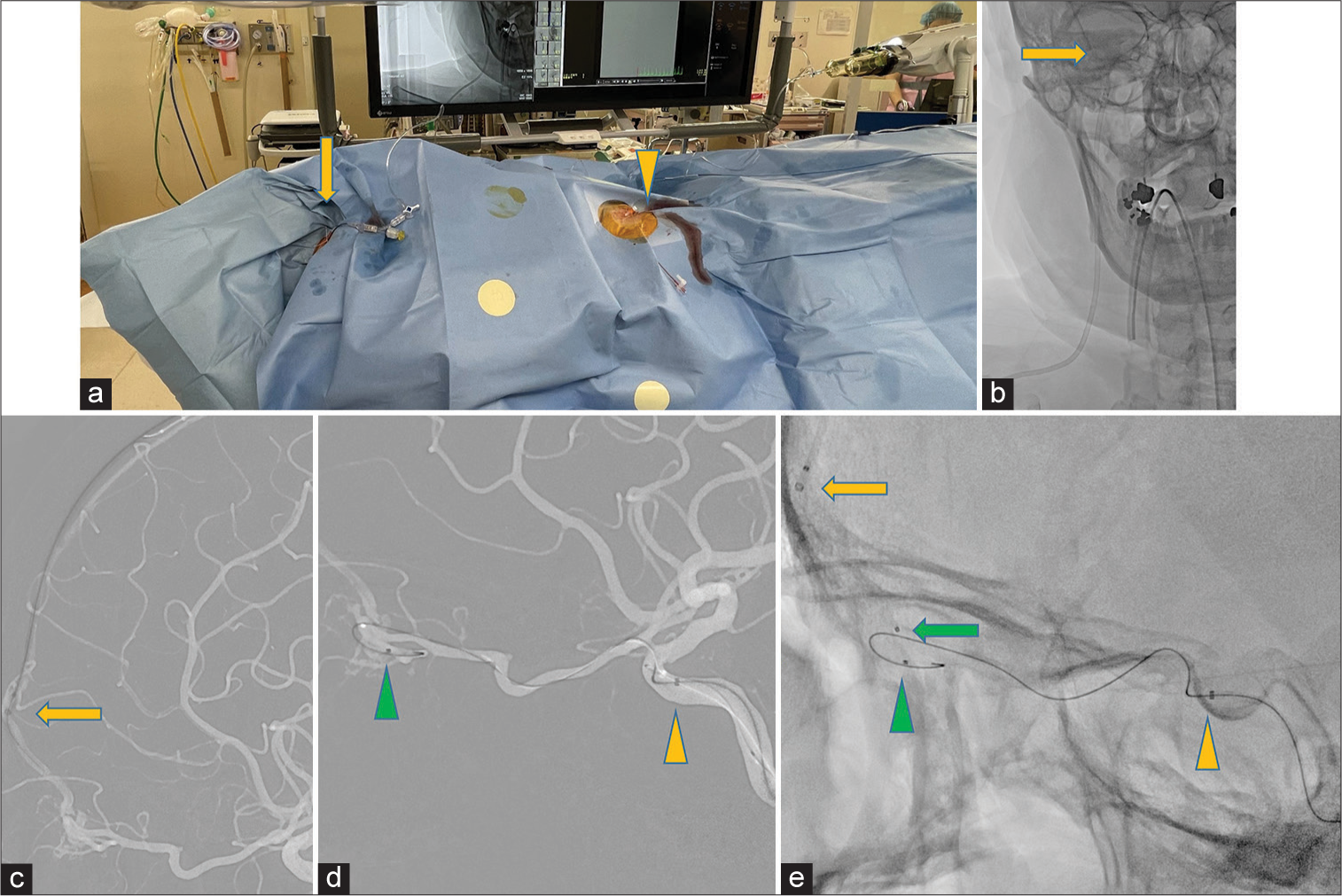
Figure 3:
(a) The procedure is performed under general anesthesia using transfemoral artery (yellow arrow head) and transvenous internal jugular vein (yellow arrow) approaches. (b) Parent Plus45 (28 cm) is placed into the right internal jugular vein (yellow arrow). (c) Retrograde transvenous access from the right internal jugular vein to the superior sagittal sinus, and an intermediate catheter TACTICS PLUS reaches to the bifurcation base of the left frontal vein (yellow arrow). (d and e) TACTICS PLUS insertion into an internal carotid artery cavernous portion (yellow arrowhead), with Marathon being advanced over a microwire to the adjacent fistulae (green arrowhead). Excelsior SL-10 being advanced over a microwire to the foot of the vein (green arrow) supported by TACTICS PLUS at the base of the left frontal vein (yellow arrow).
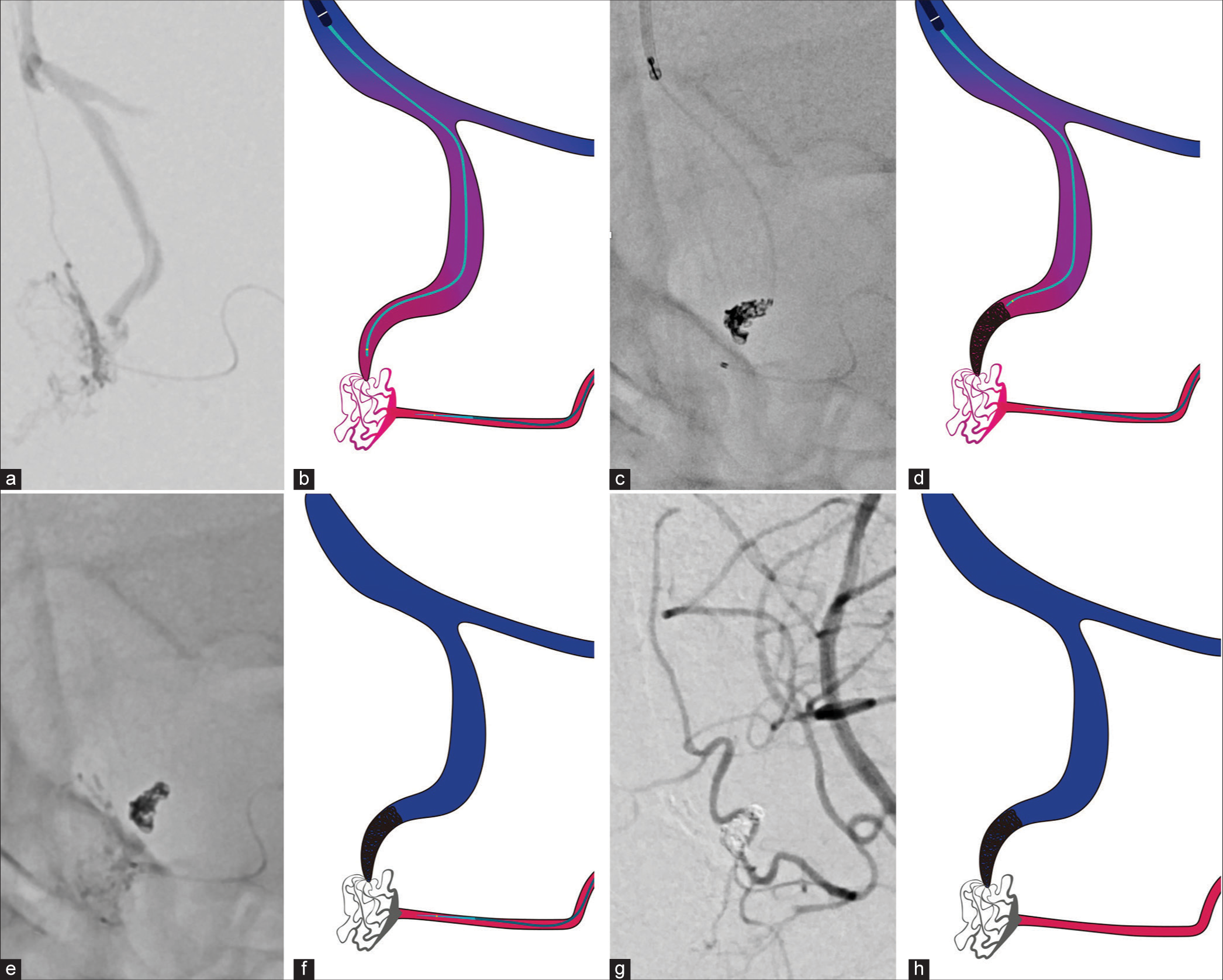
Figure 4:
A series of angiograms and illustrations during the procedure. (a and b) Selective angiogram from the microcatheter placed in the left anterior ethomoidal artery. Note the tip of the microcatheter for transvenous embolization placement near the shunt. (c and d) Coils are placed in the foot of the vein for flow control. (e-h) Low-concentration n-butyl-2-cyanoacrylate (14%) is injected from the arterial side without fragmentation into the venous drainage. Note the glue penetration of the fistulous point without extending to the confluence of the normal frontal cortical vein.
Figure 5:
(a) Frontal and (b) lateral projections of the left internal carotid artery angiogram obtained at the end of session show the complete occlusion of the anterior cranial fossa dural arteriovenous fistula. Magnified oblique view of the left internal carotid angiogram(c and d) show the complete occlusion of the shunt with the preservation of the left frontal cortical vein (yellow arrows).
DISCUSSION
Intracranial hemorrhage has been reported as the most common presentation of ACF dAVF.[
In our case, preoperative CTA revealed that the affected and normal veins in the left frontal lobe had merged to form a common trunk, and the distance from the shunt point to the confluence was <2 cm. By reducing drainage flow from the shunt by TVE with coils, it was possible to prevent the glue from extending from the drainage vein to the confluence. Coil embolization alone does not completely eliminate the reflux itself; however, reducing the flow was enough to control the glue penetration. The purpose was to provide flow control as an adjuvant to TAE.
We should also discuss the disadvantages of TVE for ACF dAVF. Precise preoperative angiographical evaluation was essential; excessively long cortical distances and tortuosity are contraindications to the use of the venous approach.[
Navigation in the tortuous vein may cause venous perforation, resulting in intracranial hemorrhage. In our case, the venous pathway was relatively short and superficial and we achieved a dense filling of the shunt while preserving normal venous perfusion in the left anterior lobe. As mentioned above, the distance to the confluence of the frontal cortical vein was within 2 cm, glue would have easily reached the confluence without coiling in the drainage vein. To the best of our knowledge, there have been no reports of TAE intentionally performed with TVE for venous flow control. Embolization can be safely and efficiently performed by pinching the shunt pouch from the arterial and venous sides. This technique is useful to form a more stable plug in the shunt and avoid migration into normal veins and sinuses.
Our study has some limitations. This was a case report from a single center. Thus, the future studies with a larger number of patients are required to validate this technique. Moreover, this method has an increased cost and angiography time. It is preferable not to use this method, particularly if the venous pathway is tortuous. Because coiling microcatheters are generally stiff for this purpose, coils cannot be used if catheterization of tortuous veins is required.
CONCLUSION
We reported a patient with ACF dAVF in whom TAE combined with TVE was useful in preserving a normal cortical vein that drained into the pathological draining vein. During the injection of the glue, this technique was effective in preventing unintended migration of the glue cast into the drainage vein, causing venous ischemia, which is a complication of TAE.
Declaration of patient consent
The authors certify that they have obtained appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abrahams JM, Bagley LJ, Flamm ES, Hurst RW, Sinson GP. Alternative management considerations for ethmoidal dural arteriovenous fistulas. Surg Neurol. 2002. 58: 410-6 discussion 6
2. Başkaya MK, Suzuki Y, Seki Y, Negoro M, Ahmed M, Sugita K. Dural arteriovenous malformations in the anterior cranial fossa. Acta Neurochir (Wien). 1994. 129: 146-51
3. Carlson AP, Taylor CL, Yonas H. Treatment of dural arteriovenous fistula using ethylene vinyl alcohol (onyx) arterial embolization as the primary modality: short-term results. J Neurosurg. 2007. 107: 1120-5
4. Davies MA, Ter Brugge K, Willinsky R, Wallace MC. The natural history and management of intracranial dural arteriovenous fistulae. Part 2: Aggressive lesions. Interv Neuroradiol. 1997. 3: 303-11
5. Halbach VV, Higashida RT, Hieshima GB, Wilson CB, Barnwell SL, Dowd CF. Dural arteriovenous fistulas supplied by ethmoidal arteries. Neurosurgery. 1990. 26: 816-23
6. Hiramatsu M, Sugiu K, Hishikawa T, Nishihiro S, Kidani N, Takahashi Y. Results of 1940 embolizations for dural arteriovenous fistulas: Japanese Registry of Neuroendovascular Therapy (JR-NET3). J Neurosurg. 2019. p. 1-8
7. Kikuchi K, Kowada M. Anterior fossa dural arteriovenous malformation supplied by bilateral ethmoidal arteries. Surg Neurol. 1994. 41: 56-64
8. Kulanthaivelu K, Pendharkar H, Prasad C, Gupta AK, Ramalingaiah AH, Saini J. Anterior cranial fossa dural arteriovenous fistulae-angioarchitecture and intervention. Clin Neuroradiol. 2021. 31: 661-9
9. Lawton MT, Chun J, Wilson CB, Halbach VV. Ethmoidal dural arteriovenous fistulae: An assessment of surgical and endovascular management. Neurosurgery. 1999. 45: 805-10 discussion 10-1
10. Li C, Wu Z, Yang X, Li Y, Jiang C, He H. Transarterial treatment with onyx of cognard Type IV anterior cranial fossa dural arteriovenous fistulas. J Neurointerv Surg. 2014. 6: 115-20
11. Limbucci N, Leone G, Nappini S, Rosi A, Renieri L, Consoli A. Transvenous embolization of ethmoidal dural arteriovenous fistulas: Case series and review of the literature. World Neurosurg. 2018. 110: e786-93
12. Martin NA, King WA, Wilson CB, Nutik S, Carter LP, Spetzler RF. Management of dural arteriovenous malformations of the anterior cranial fossa. J Neurosurg. 1990. 72: 692-7
13. Piergallini L, Tardieu M, Cagnazzo F, Gascou G, Dargazanli C, Derraz I. Anterior cranial fossa dural arteriovenous fistula: Transarterial embolization from the ophthalmic artery as first-line treatment. J Neuroradiol. 2021. 48: 207-14
14. Pierot L, Visot A, Boulin A, Dupuy M. Combined neurosurgical and neuroradiological treatment of a complex superior sagittal sinus dural fistula: Technical note. Neurosurgery. 1998. 42: 194-7
15. Reul J, Thron A, Laborde G, Brückmann H. Dural arteriovenous malformations at the base of the anterior cranial fossa: Report of nine cases. Neuroradiology. 1993. 35: 388-93
16. Robert T, Blanc R, Smajda S, Ciccio G, Redjem H, Bartolini B. Endovascular treatment of cribriform plate dural arteriovenous fistulas: Technical difficulties and complications avoidance. J NeuroInterv Surg. 2016. 8: 954-8
17. Ros de San Pedro J, Pérez CJ, Parra JZ, López-Guerrero AL, Sánchez JF. Bilateral ethmoidal dural arteriovenous fistula: Unexpected surgical diagnosis. Clin Neurol Neurosurg. 2010. 112: 903-8
18. Russell SM, Woo HH, Nelson PK. Transarterial wedged-catheter, flow-arrest, N-butyl cyanoacrylate embolization of three dural arteriovenous fistulae in a single patient. Interv Neuroradiol. 2003. 9: 283-90
19. Trivelato FP, Smajda S, Saleme S, Castro-Afonso LH, Abud TG, Ulhôa AC. Endovascular treatment of anterior cranial base dural arteriovenous fistulas as a first-line approach: A multicenter study. J Neurosurg. 2022. 137: 1758-65
20. Xu K, Ji T, Li C, Yu J. Current status of endovascular treatment for dural arteriovenous fistulae in the anterior cranial fossa: A systematic literature review. Int J Med Sci. 2019. 16: 203-11