- Department of Neurosurgery, Jinnah Sindh Medical University, Karachi, Sindh, Pakistan
- Department of Neurosurgery, Ziauddin University, Karachi, Sindh, Pakistan
- Department of Neurosurgery, Liaquat National Hospital and Medical College, Karachi, Sindh, Pakistan.
Correspondence Address:
Muhammad Ashir Shafique, Department of Neurosurgery, Jinnah Sindh Medical University, Karachi, Sindh, Pakistan.
DOI:10.25259/SNI_994_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Burhanuddin Sohail Rangwala1, Tooba Noor1, Areej Shakil1, Muhammad Saqlain Mustafa1, Muhammad Ashir Shafique1, Sadia Manan2, Amna Qamber1, Syeda Dua E Zehra Zaidi1, Muhammad Adil Obaid1, Irja Munawar1, Sabah Rizvi3, Hussain Sohail Rangwala1. Comparing equiosmolar hypertonic saline and mannitol for achieving brain relaxation in elective craniotomy patients: A systematic review and meta-analysis. 05-Apr-2024;15:116
How to cite this URL: Burhanuddin Sohail Rangwala1, Tooba Noor1, Areej Shakil1, Muhammad Saqlain Mustafa1, Muhammad Ashir Shafique1, Sadia Manan2, Amna Qamber1, Syeda Dua E Zehra Zaidi1, Muhammad Adil Obaid1, Irja Munawar1, Sabah Rizvi3, Hussain Sohail Rangwala1. Comparing equiosmolar hypertonic saline and mannitol for achieving brain relaxation in elective craniotomy patients: A systematic review and meta-analysis. 05-Apr-2024;15:116. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12847
Abstract
Background: This study strives to provide a current and thorough assessment of the comparative efficacy and safety between equiosmolar quantities of hypertonic saline (HS) and mannitol in facilitating brain relaxation for patients undergoing elective craniotomies.
Methods: This systematic review and meta-analysis, following preferred reporting items for systematic reviews and meta-analyses guidelines, compared the efficacy and safety of equiosmolar concentrations of mannitol and HS in elective craniotomies. PubMed, Scopus, Cochrane Library, ScienceDirect, and Proquest databases were searched using keywords related to mannitol, HS, and craniotomy. Results were analyzed through a random-effects model using Mantel–Haenszel risk ratio and standard mean difference. P
Results: Thirteen randomized controlled trials encompassing 965 patients (516 in the HS group and 448 in the mannitol group) were analyzed. The quality of studies was moderate-to-high, and no significant publication bias was observed. The primary outcome, brain relaxation, favored HS over mannitol without significant heterogeneity. Mannitol was associated with increased urine output compared to HS, irrespective of dose, with high heterogeneity. HS was linked to significantly reduced fluid input, confirmed by subgroup analysis with lower heterogeneity. No significant difference was found in serum osmolality between the two agents. Serum sodium (Na+) levels favored HS, whereas arterial blood Na+ levels also favored HS despite considerable heterogeneity. Maximum mean arterial pressure was higher with HS, but it displayed significant heterogeneity. Maximum central venous pressure showed no significant difference between the two agents, with moderate heterogeneity.
Conclusion: HS appears more effective than mannitol in achieving brain relaxation, and it may offer advantages in fluid management and Na+ balance. Clinicians should consider these findings when selecting hyperosmotic agents for neurosurgical procedures. Further research is needed to address heterogeneity in certain outcomes and guide clinical practice.
Keywords: Brain relaxation, Craniotomy, Hypertonic saline, Mannitol
INTRODUCTION
Cerebral relaxation is a crucial neuroprotective measure to mitigate local hypoperfusion and cerebral ischemia due to surgical compression following intracranial surgery; therefore, achieving a relaxed brain is a fundamental goal in neurosurgical anesthesia.[
During a neurosurgical procedure, osmotherapy is routinely utilized to lower intracranial pressure (ICP) and the volume of brain tissue, for which hyperosmotic drugs such as mannitol and hypertonic saline (HS) are delivered before the opening of dura mater, reducing the risk of transdural herniation and associated neurological deterioration.[
Mannitol is the preferred hyperosmotic medication, offering both immediate and delayed reductions in ICP.[
Notably, in comparison to mannitol, HS exerts its impact by establishing an osmotic gradient, leading to brain tissue dehydration and cerebral vasoconstriction, ultimately reducing ICP. It has recently rekindled attention as an alternative therapy and has lately been employed in neurosurgical patients because of its additional positive effects that promote gas exchange and partial pressure of oxygen in the blood, including an increase in cardiac output, a decrease in extravascular lung capacity, and an increase in mean arterial pressure (MAP).[
Clinical research comparing the impact of HS and mannitol on ICP has indicated that HS is, at the very least, as effective as, if not more effective than, mannitol in addressing elevated ICP.[
MATERIALS AND METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards were followed for conducting this systematic review and meta-analysis.[
Search strategy
We searched extensively through a number of databases, including PubMed, Scopus, Cochrane Library, ScienceDirect, and Proquest, to find randomized controlled trials (RCTs) that contrasted the effects of mannitol and HS at equiosmolar concentrations on patients undergoing elective craniotomies. To get relevant results, In August 2023, we conducted a search using both Medical Subjects Headings phrases and specific keywords such as “mannitol,” “hypertonic saline,” “brain relaxation,” “osmotherapy,” “craniotomy,” and “neurosurgery.” No additional restrictions were applied to the search criteria.
Study selection and eligibility criteria
Two reviewers (AS and IM) conducted an independent search and screening of journals. The inclusion criteria for our study were as follows: (1) Only RCTs were considered; (2) studies had to involve the use of equal osmolar amounts of HS as compared to mannitol; (3) participants should be patients undergoing elective craniotomies for a range of brain conditions; (4) the studies needed to report relevant outcomes; (5) the documentation had to be in English; and (6) the full text of the studies had to be accessible.
Data extraction and quality assessment
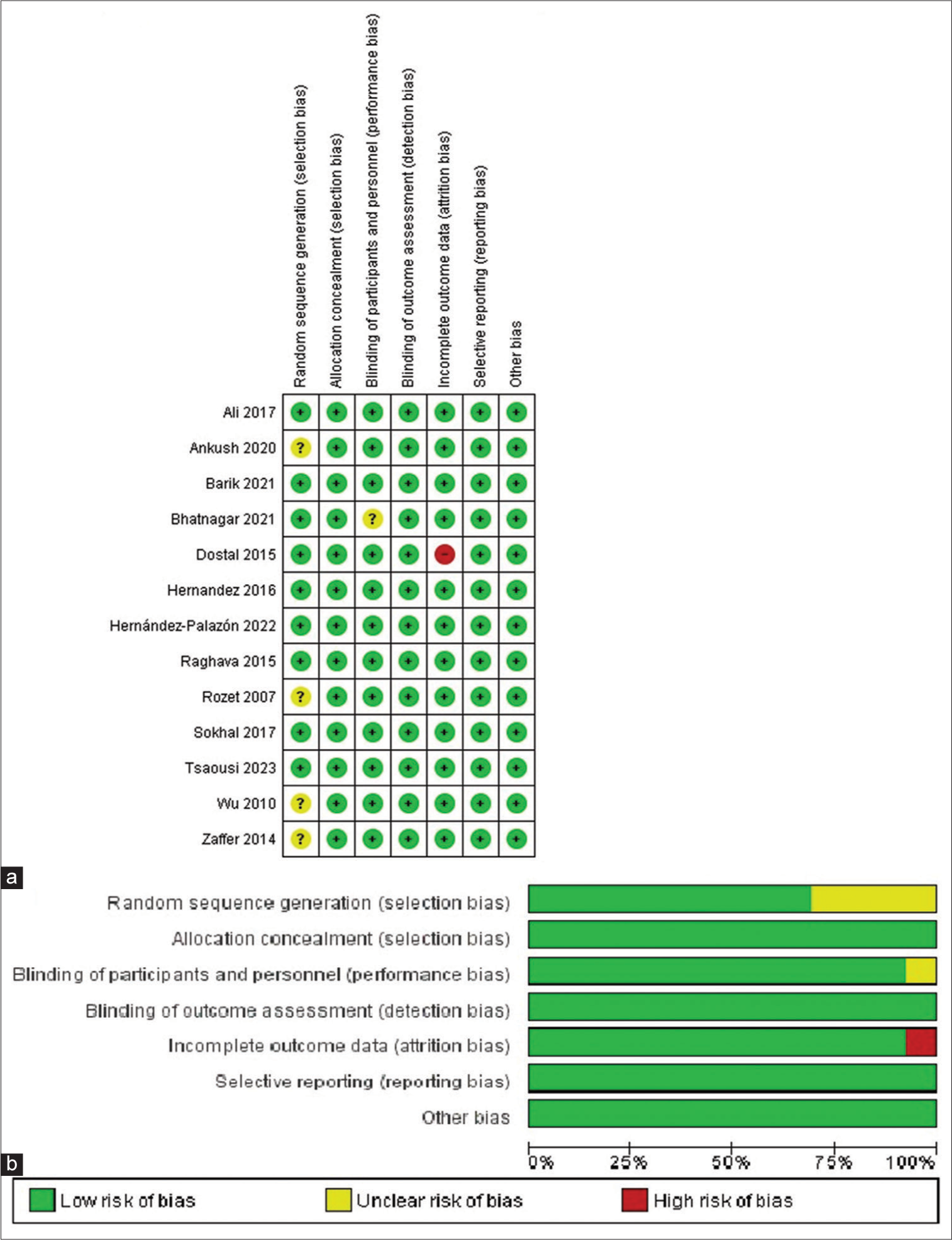
Two authors (MAS and MSM) separately gathered the required data, and any discrepancies were resolved through discussion and the involvement of a third review author (BSR). Two investigators (BSR and MAO) individually evaluated the study quality and bias risk of each research study. They used the Cochrane Risk of Bias tool specifically for RCT.[
Data analysis
Examining the effects of brain relaxation was the main focus of our observation. The degree of brain relaxation was measured using a 4-point scale that went from “perfectly relaxed” to “bulging brain” or a 3-point scale that included “tight,” “appropriate,” and “soft.” We used judgments of “perfectly relaxed,” “satisfactory relaxed,” “appropriate,” and “soft” as markers of good brain relaxation to produce binary results. To evaluate this main outcome, the odds ratio and associated 95% confidence intervals (CIs) were used.
The secondary outcomes we took into account included the following: Total urine output and fluid intake were assessed using standardized mean difference (MD) with a 95% CI; serum sodium (na), arterial sodium, plasma osmolality, maximum MAP, and maximum central venous pressure (CVP) were assessed using MD with a 95% CI. To further investigate the relationship between total urine production and various dosage levels, we also performed subgroup analyses.
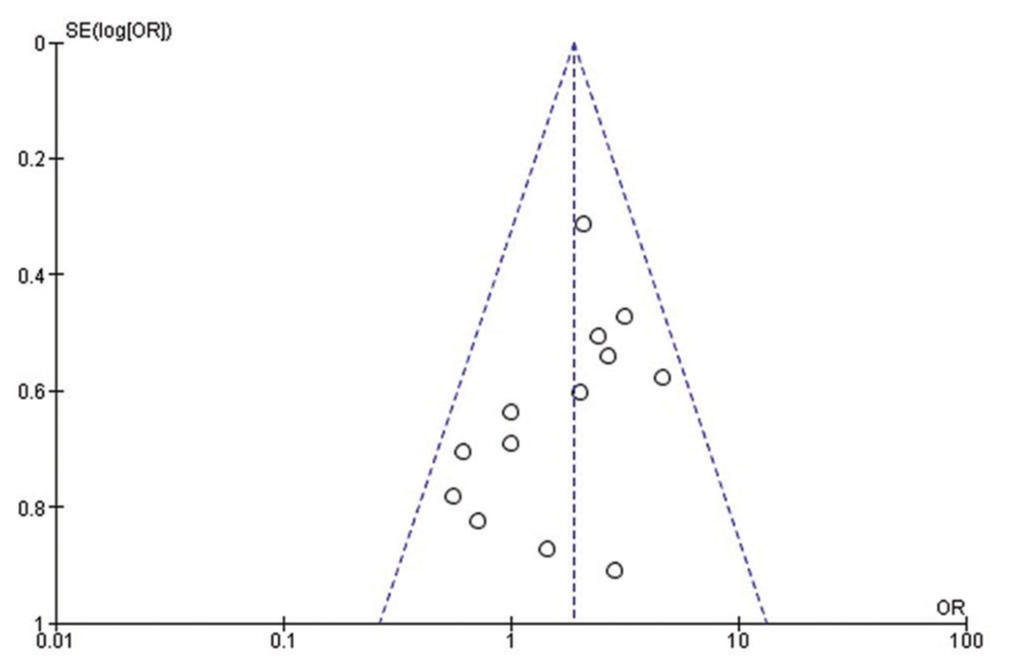
A significance level of P < 0.05 was considered statistically significant, and the analysis incorporated 95% CIs. Sensitivity analyses were initiated by excluding potentially questionable studies to identify sources of heterogeneity, which was gauged using I2 and the P-value derived from the Chi-square test; for outcomes displaying considerable heterogeneity, characterized by an I2 value surpassing 50%, a random-effects model was employed. The meta-analysis and generation of forest plots were performed using Review Manager version 5.4. In addition, potential publication bias was assessed through a visual inspection of the funnel plot.
RESULTS
Literature search results study characteristics
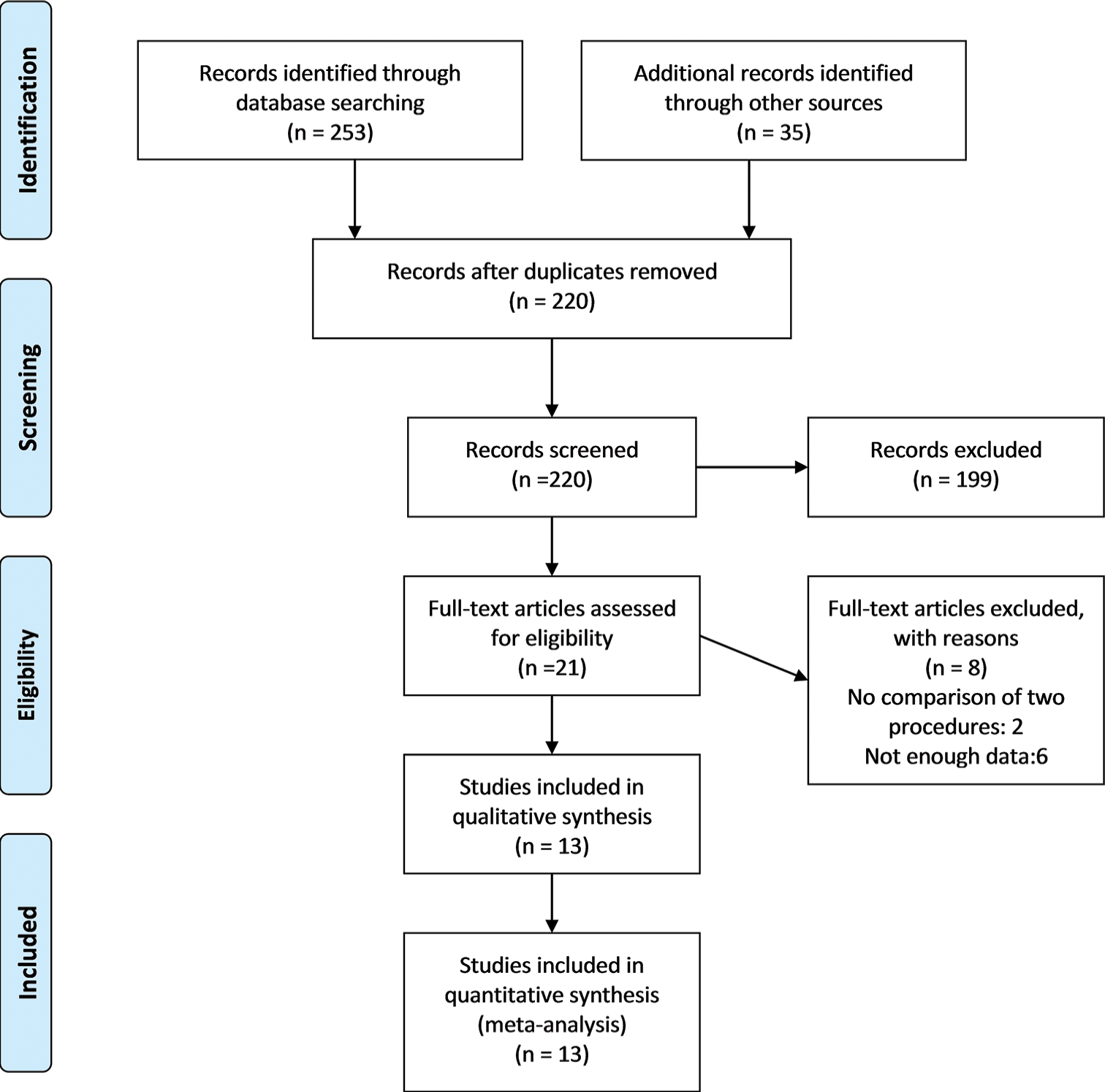
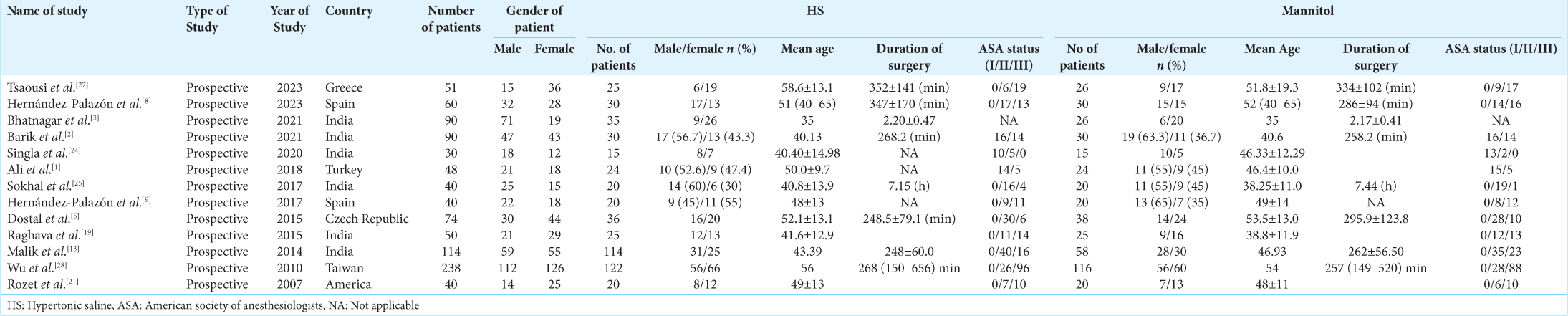
We initially found a total of 253 articles. We examined the titles and abstracts of the remaining papers and included 21 studies after deleting 33 duplicate research. We carefully examined 18 full-text studies. Out of them, our study incorporated the most recent 13 RCTs that were published between 2007 and 2023, as depicted in
Quality assessment and publication bias
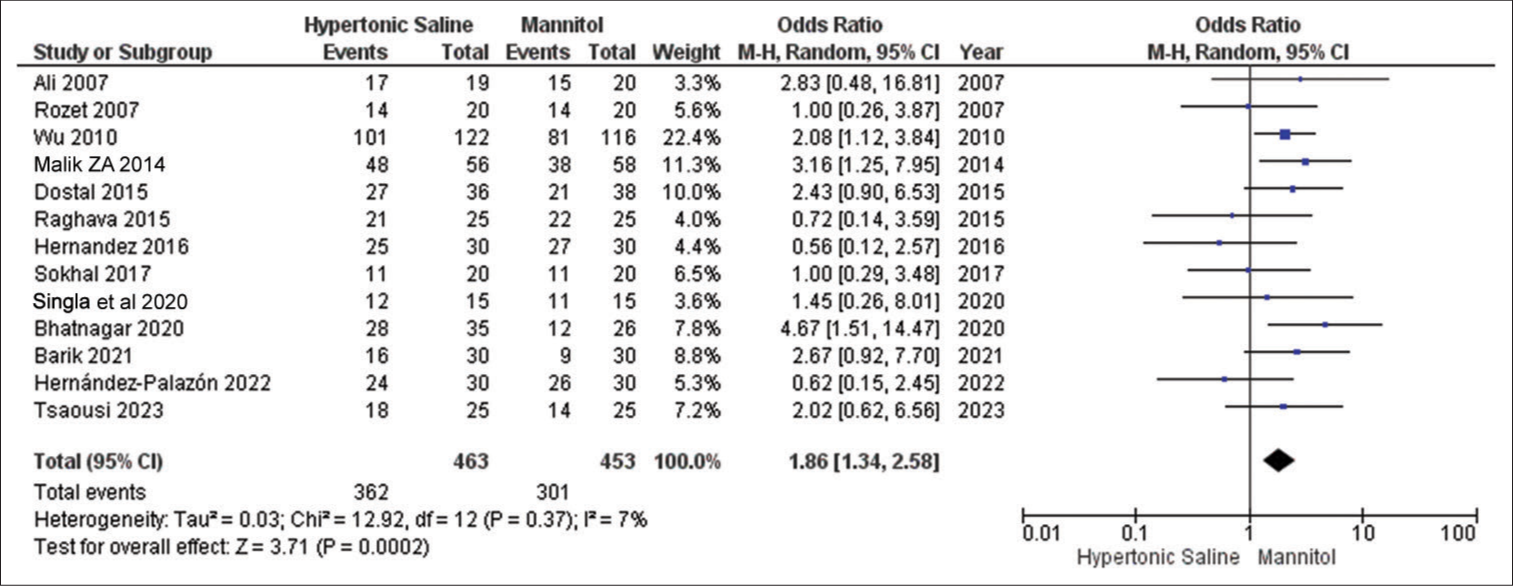
Brain relaxation
In all 13 studies, this particular outcome was reported, involving a total of 936 patients. The results consistently show that HS is significantly more effective than mannitol in achieving improved brain relaxation scores. Notably, there was no notable variation observed in the outcomes across these studies [
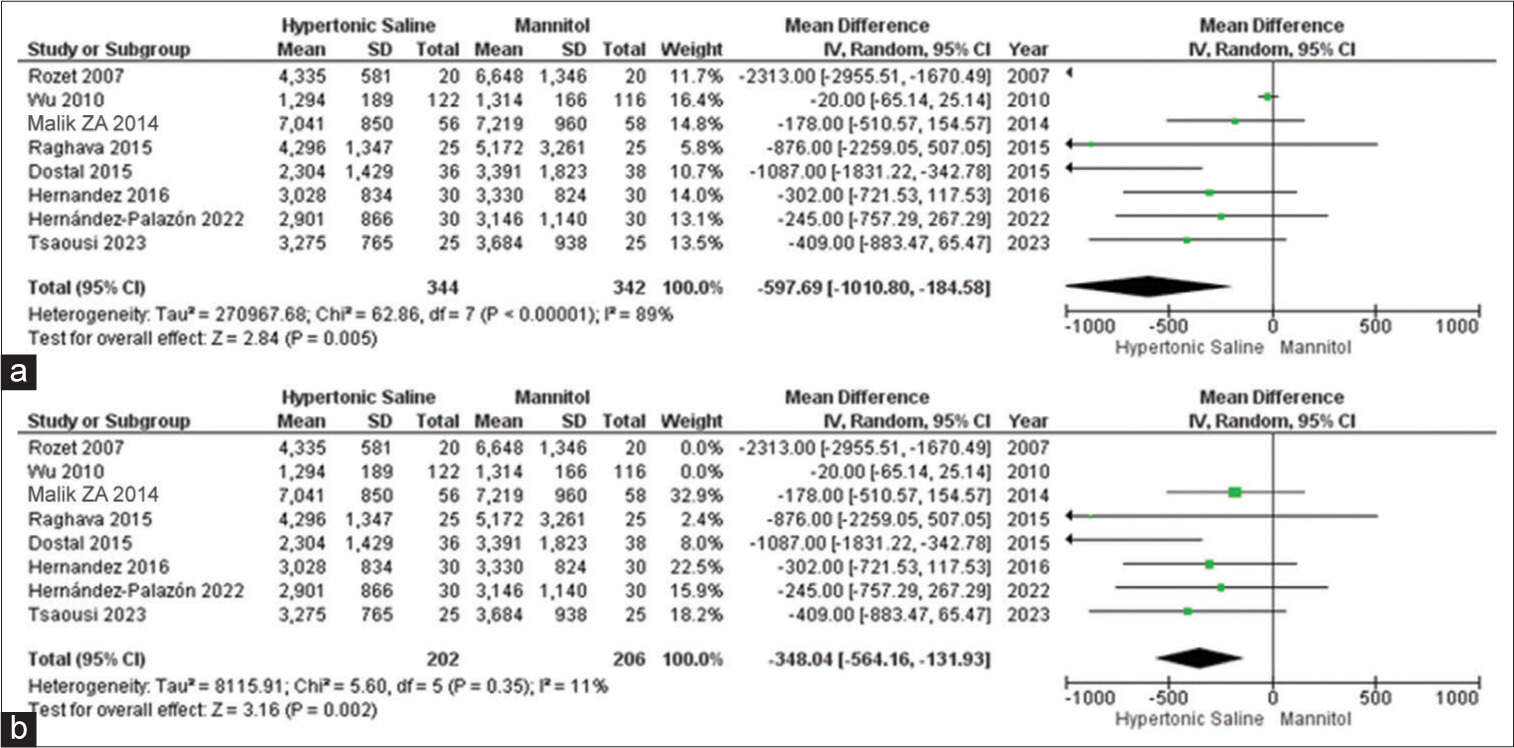
Urine output
Out of the 13 studies, 9 of them provided data on urine output, involving a total of 726 patients. Mannitol was found to be linked to a noteworthy increase in total urine output when compared to HS. Due to the observed high heterogeneity, we conducted a sensitivity analysis to pinpoint the source of this variation. The subgroup analysis demonstrated that irrespective of the dosage administered, there was a substantial increase in urine output when using mannitol as opposed to HS [
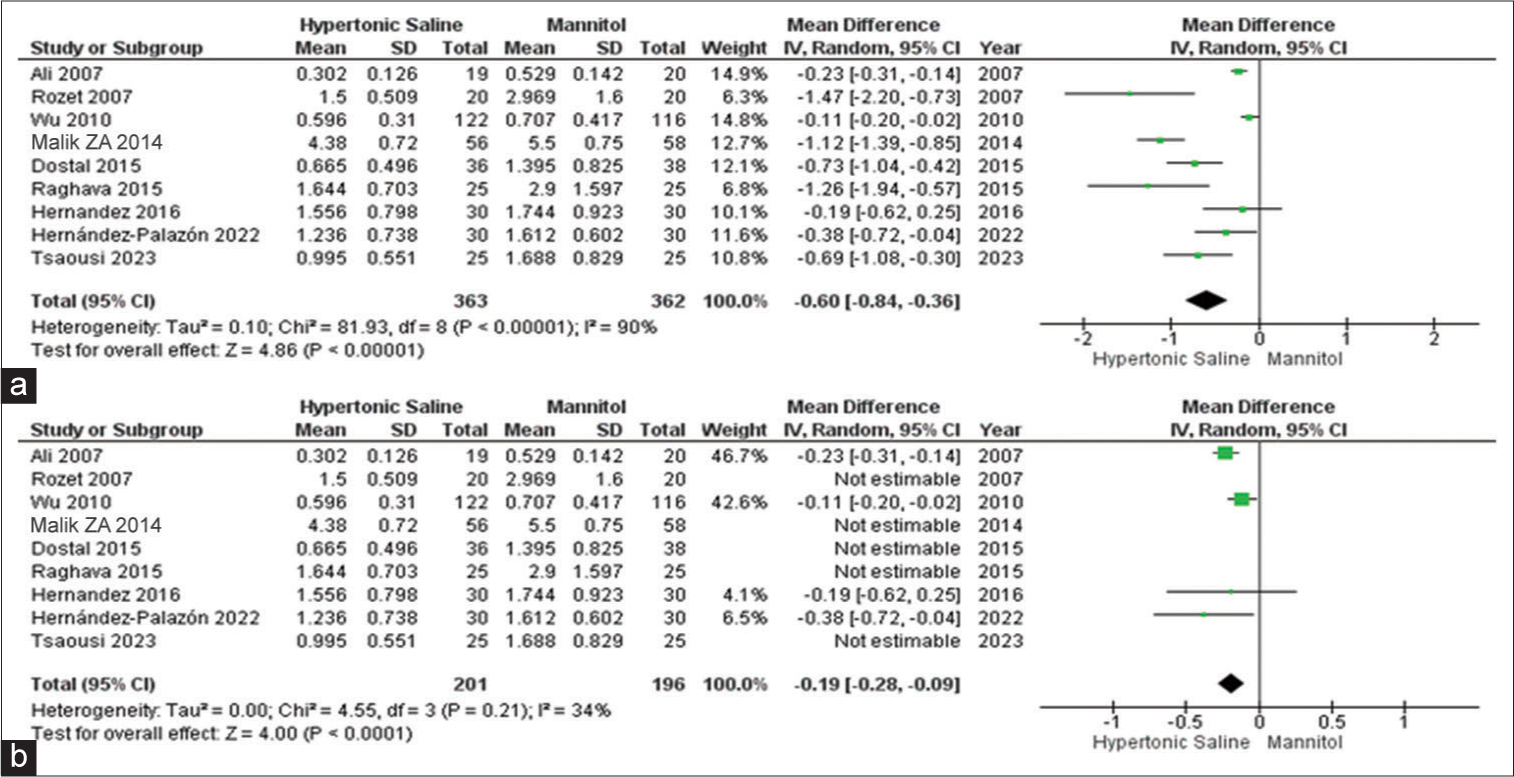
Fluid input
Among the complete set of 13 studies, eight of them presented data on fluid input, encompassing a total of 687 patients. It was evident that HS was linked to notably reduced total fluid input when contrasted with mannitol. Due to the observed high heterogeneity, a subgroup analysis was conducted, which corroborated the previous findings but with significantly reduced heterogeneity [
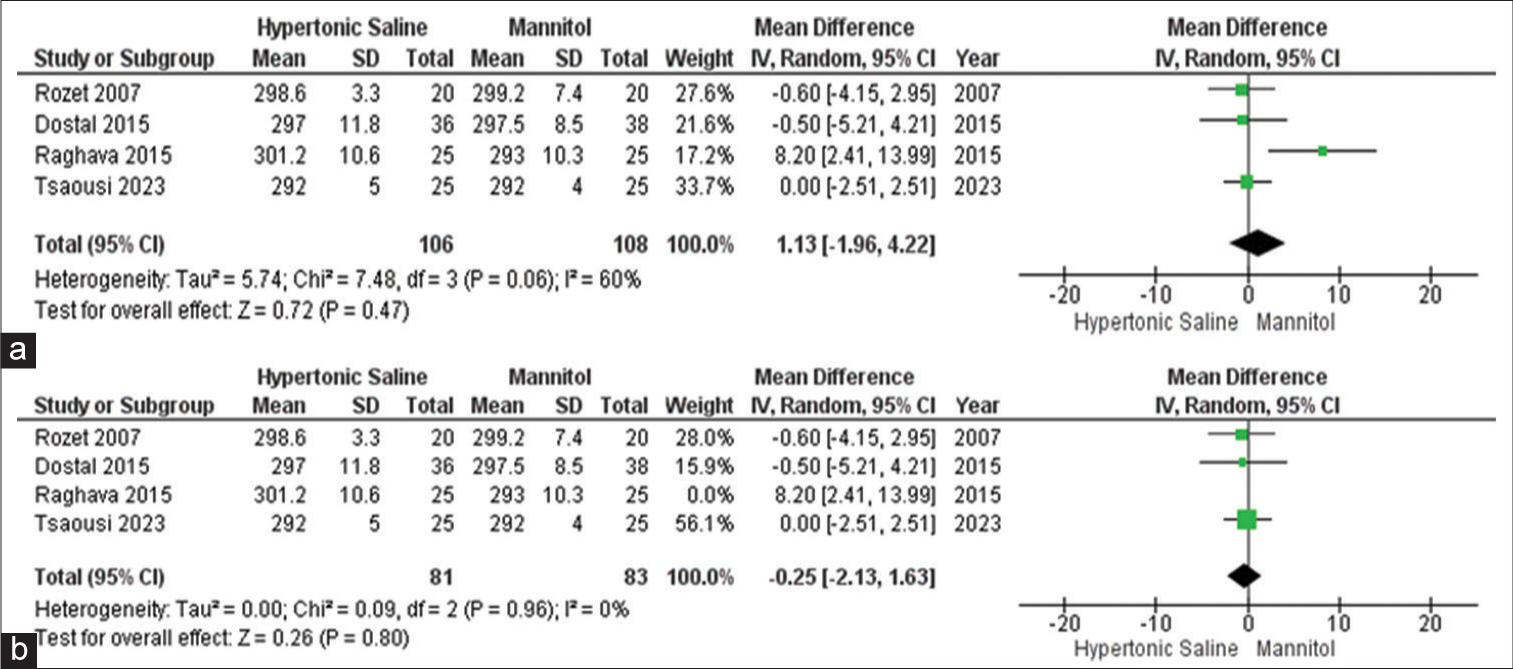
Serum osmolality
Out of all the studies, only four of them provided information on this particular outcome, involving a total of 215 participants. The data did not reveal any significant distinction in serum osmolality when comparing the use of mannitol and HS in elective craniotomies. To pinpoint the origin of the high heterogeneity observed, a sensitivity analysis was conducted, further confirming that there is no disparity in serum osmolality between mannitol and HS [
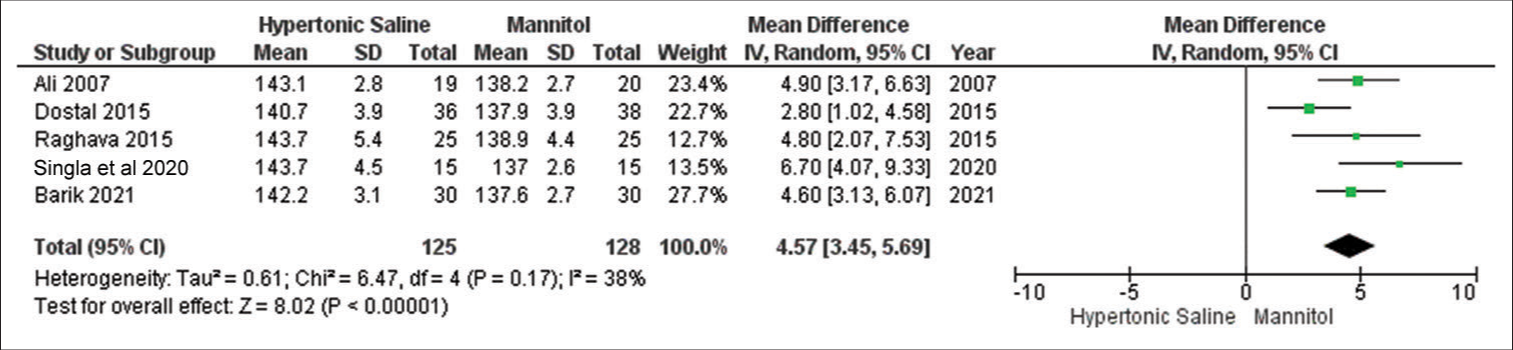
Serum Na+
In the analysis, data from a subset of five out of 13 studies were considered, encompassing a total of 253 sampled patients. The findings suggest that HS exhibited favorable outcomes for brain relaxation in comparison to mannitol. Notably, there was no noteworthy evidence of significant heterogeneity among the included studies [
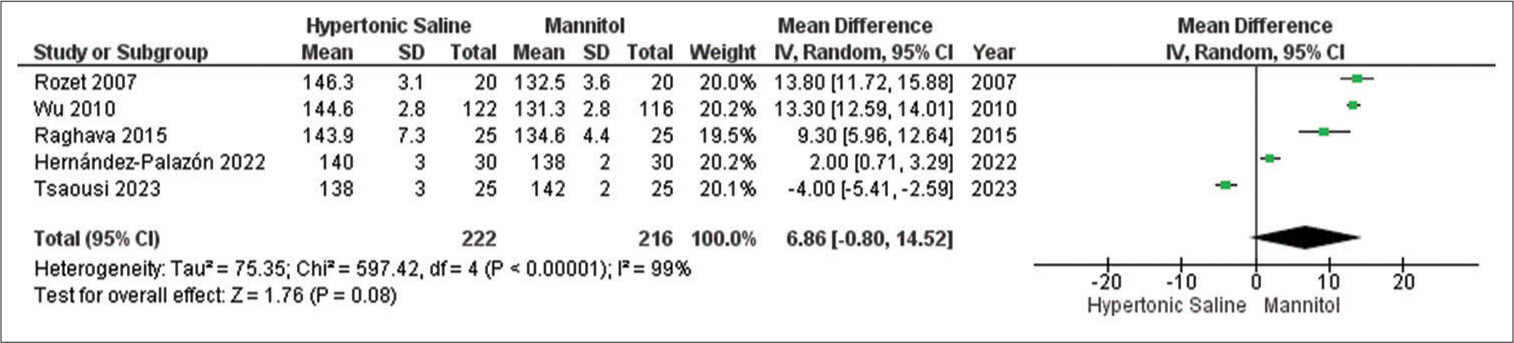
Arterial blood Na+ levels
Out of the studies analyzed, only five studies provided data on the outcome, involving a combined sample size of 439 patients. Mannitol exhibited notably lower relaxation scores compared to HS, even in the presence of considerable heterogeneity across the studies [
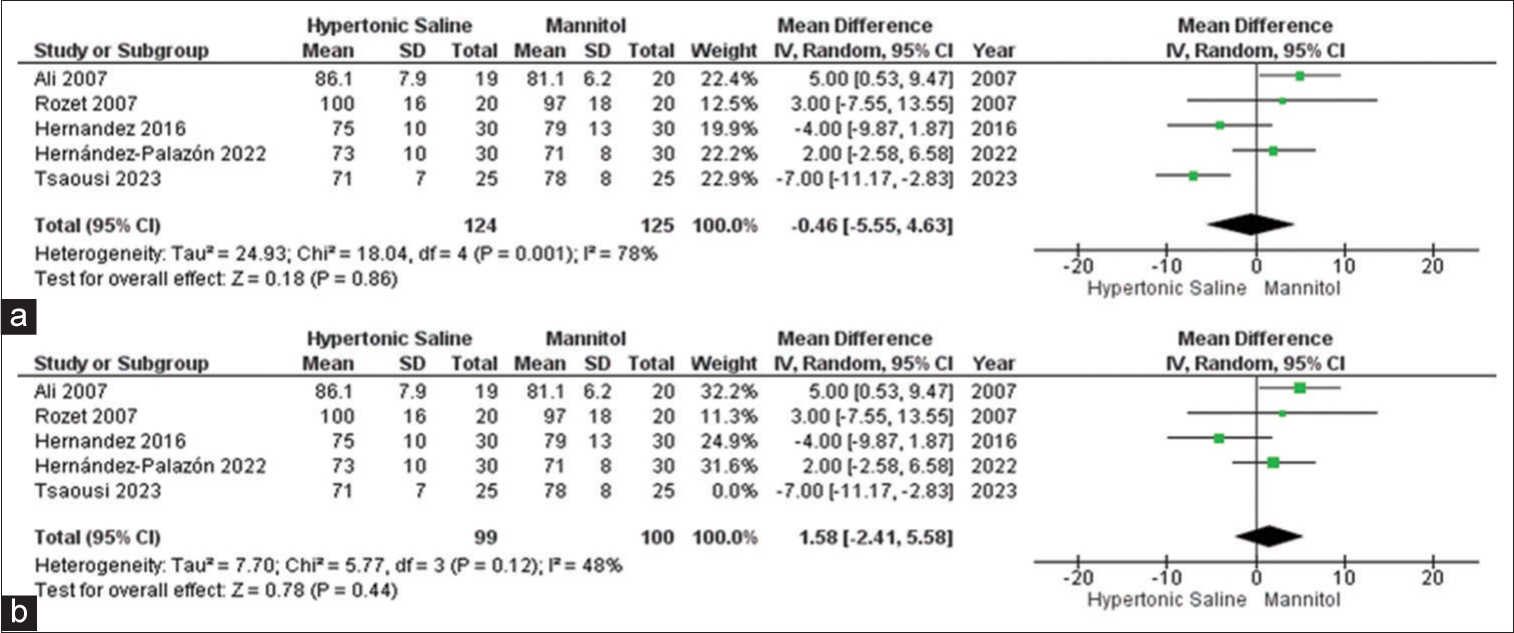
Max MAP
In the analysis, data from a subset of five out of the 13 studies were considered, encompassing a total of 253 sampled patients. The findings suggest that HS exhibited favorable outcomes for brain relaxation in comparison to mannitol. Notably, there was evidence of significant heterogeneity among the included studies [
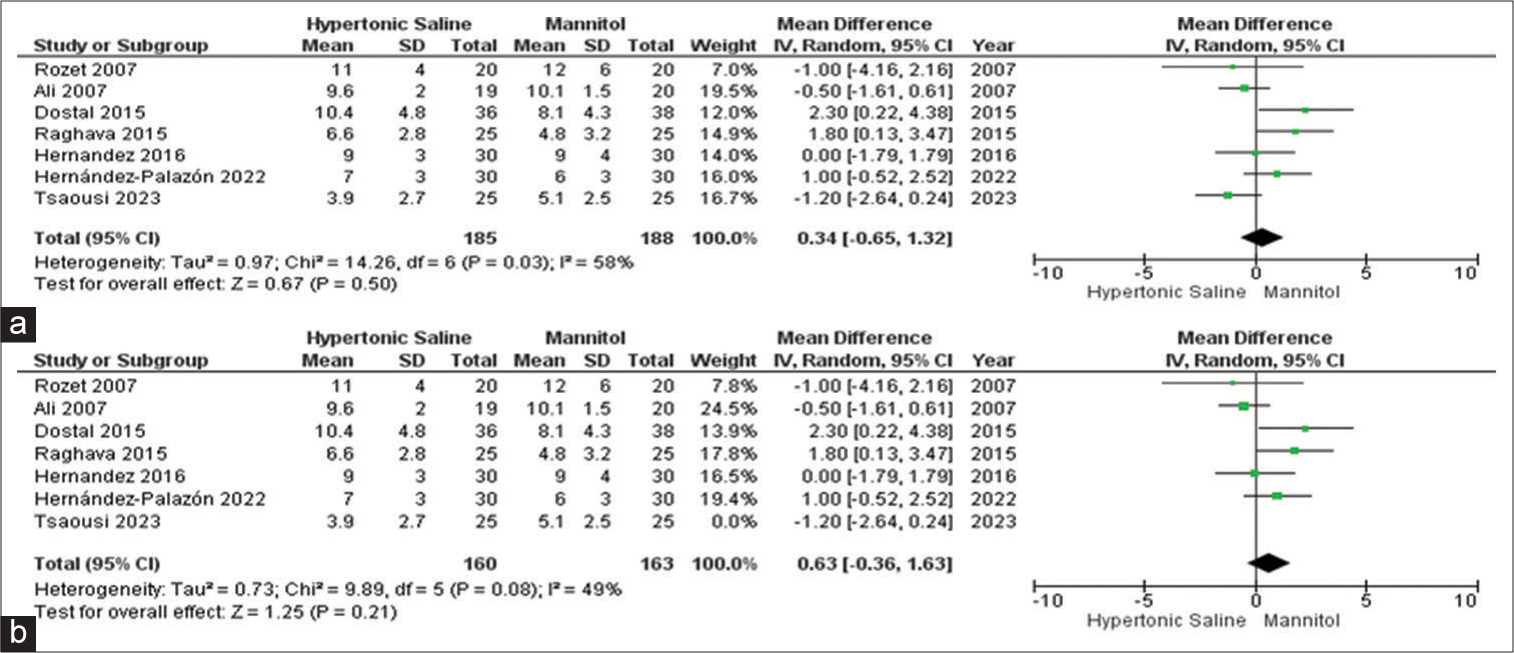
Max CVP
Among the set of 13 included studies, seven studies provided data on the outcome related to maximum CVP, involving a collective sample size of 374 patients. No significant outcomes were identified for either HS or mannitol. To better understand the source of observed high heterogeneity, a sensitivity analysis was conducted, which reaffirmed a slightly more favorable score associated with HS, accompanied by a moderate level of heterogeneity [
DISCUSSION
This meta-analysis is an update on Theodorus’ previous meta-analysis in 2022.[
However, the three new studies, we included revealed that the max MAP and max CVP were higher for HS as compared to mannitol.[
Our meta-analysis revealed that HS is linked to a notably higher brain relaxation score in comparison to mannitol which is in line with the result of the previous meta-analysis.[
Just like mannitol, its primary mode of action involves generating an osmotic gradient across the blood-brain barrier (BBB), leading to a shift in cerebral fluid and a reduction in ICP and cerebral swelling.[
The findings from this meta-analysis indicated that HS was linked to elevated plasma Na+ levels and exhibited a comparatively reduced diuretic efficacy when contrasted with mannitol proven by reduced urine output. Elevated serum Na+ levels trigger the production of antidiuretic hormones, resulting in absorption of free water by the kidneys resulting in lower urine output by HS.[
The potent diuretic effect of mannitol can lead to hypovolemia, an undesirable condition during neurosurgical procedures for which surgeons should be aware. In addition to this, Rozet et al. concluded higher serum lactate levels as a result of diuresis in patients treated with mannitol.[
Based on multiple previous meta-analyses, the most frequently observed adverse outcomes are related to these electrolyte imbalances, such as hypernatremia, which can prove to be significant in patients with electrolyte abnormalities.[
Incorporating three new studies into our analysis showed that HS consistently raised both maximum MAP and maximum CVP compared to mannitol. This is particularly valuable in elective craniotomies, where maintaining adequate cerebral perfusion pressure (CPP) is crucial. Higher MAP levels achieved with HS can help maintain the desired CPP range, reducing the risk of cerebral ischemia during surgery. In addition, HS has a greater propensity to increase CVP when compared to mannitol. While an elevated CVP can be beneficial in specific situations, such as managing ICP in patients with intracranial hypertension, it may present challenges in patients with compromised cardiac function. On the other hand, mannitol demonstrated a relatively minor impact on CVP, which could be advantageous in cases prioritizing the avoidance of fluid overload and excessive CVP elevation.[
CONCLUSION
Our updated systematic review and meta-analysis indicate that equiosmolar doses of HS outperform mannitol in terms of brain relaxation in patients undergoing elective craniotomies. However, the selection of the osmotic agent should be based on the patient’s clinical condition, safety considerations, and surgical objectives. Further research and long-term studies are needed to refine our understanding of the comparative effectiveness of these osmotic agents and their impact on patient outcomes in neurosurgery.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ali A, Tetik A, Sabanci PA, Altun D, Sivrikoz N, Abdullah T. Comparison of 3% hypertonic saline and 20% mannitol for reducing intracranial pressure in patients undergoing supratentorial brain tumor surgery: A randomized, double-blind clinical trial. J Neurosurg Anesthesiol. 2018. 30: 171-8
2. Barik AK, Agrawal S, Gupta P, Kumari R. Evaluation of equiosmolar 20% mannitol, 3% hypertonic saline and 8.4% sodium bicarbonate on intraoperative brain relaxation and hemodynamic parameters in patients undergoing craniotomy for supratentorial tumors: A prospective randomized study. Minerva Anestesiol. 2021. 87: 997-1005
3. Bhatnagar N, Bhateja S, Jeenger L, Mangal G, Gupta S. Effects of two different doses of 3% hypertonic saline with mannitol during decompressive craniectomy following traumatic brain injury: A prospective, controlled study. J Anaesthesiol Clin Pharmacol. 2021. 37: 523-8
4. da Silva JC, de Lima FM, Valença MM, de Azevedo Filho HR. Hypertonic saline more efficacious than mannitol in lethal intracranial hypertension model. Neurol Res. 2010. 32: 139-43
5. Dostal P, Dostalova V, Schreiberova J, Tyll T, Habalova J, Cerny V. A comparison of equivolume, equiosmolar solutions of hypertonic saline and mannitol for brain relaxation in patients undergoing elective intracranial tumor surgery: A randomized clinical trial. J Neurosurg Anesthesiol. 2015. 27: 51-6
6. Fang J, Yang Y, Wang W, Liu Y, An T, Zou M. Comparison of equiosmolar hypertonic saline and mannitol for brain relaxation during craniotomies: A meta-analysis of randomized controlled trials. Neurosurg Rev. 2018. 41: 945-56
7. Hans P, Bonhomme V. Why we still use intravenous drugs as the basic regimen for neurosurgical anaesthesia. Curr Opin Anaesthesiol. 2006. 19: 498-503
8. Hernández-Palazón J, Doménech-Asensi P, Fuentes-García D, Burguillos-López S, Piqueras-Pérez C, García-Palenciano C. Comparison of 20% mannitol and 3% hypertonic saline for intraoperative brain relaxation during supratentorial brain tumour craniotomy in patients with a midline shift. Neurocirugia (Astur: Engl Ed). 2023. 34: 273-82
9. Hernández-Palazón J, Fuentes-García D, Doménech-Asensi P, Piqueras-Pérez C, Falcón-Araña L, Burguillos-López S. Equiosmolar solutions of hypertonic saline and mannitol do not impair blood coagulation during elective intracranial surgery. J Neurosurg Anesthesiol. 2017. 29: 8-13
10. Higgins JThomas JChandler JCumpston MLi TPage M. Cochrane handbook for systematic reviews of interventions. Available from: https://training.cochrane.org/handbook [Last accessed on 2023 Oct 23].
11. Himmelseher S. Hypertonic saline solutions for treatment of intracranial hypertension. Curr Opin Anaesthesiol. 2007. 20: 414-26
12. Johansyah TK, Jonathan J, Yusari IG, Nolan J, Alamsyah AH, Ramadhana GA. Equiosmolar doses of hypertonic saline versus mannitol for brain relaxation in patients undergoing elective craniotomies: An updated systematic review and meta-analysis. Egypt J Neurol Psychiatry Neurosurg. 2022. 58: 142
13. Malik ZA, Mir SA, Naqash IA, Sofi KP, Wani AA. A prospective, randomized, double blind study to compare the effects of equiosmolar solutions of 3% hypertonic saline and 20% mannitol on reduction of brain-bulk during elective craniotomy for supratentorial brain tumor resection. Anesth Essays Res. 2014. 8: 388-92
14. McAlister V, Burns KE, Znajda T, Church B. Hypertonic saline for peri-operative fluid management. Cochrane Database Syst Rev. 2010. 1: CD005576
15. Mishra C, Rajan BG, Sethi A, Narang N. Effect of 3% hypertonic saline and mannitol on brain relaxation during supratentorial brain tumor surgery. Int J Sci Study. 2016. 3: 154-7
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009. 6: e1000097
17. Oddo M, Levine JM, Frangos S, Carrera E, Maloney-Wilensky E, Pascual JL. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatry. 2009. 80: 916-20
18. Ogden AT, Mayer SA, Connolly ES. Hyperosmolar agents in neurosurgical practice: The evolving role of hypertonic saline. Neurosurgery. 2005. 57: 207-15
19. Raghava A, Bidkar PU, Prakash MV, Hemavathy B. Comparison of equiosmolar concentrations of hypertonic saline and mannitol for intraoperative lax brain in patients undergoing craniotomy. Surg Neurol Int. 2015. 6: 73
20. Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009. 65: 1035-41
21. Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007. 107: 697-704
22. Shao L, Hong F, Zou Y, Hao X, Hou H, Tian M. Hypertonic saline for brain relaxation and intracranial pressure in patients undergoing neurosurgical procedures: A meta-analysis of randomized controlled trials. PLoS One. 2015. 10: e0117314
23. Shao L, Wang B, Wang S, Mu F, Gu K. Comparison of 7.2% hypertonic saline-6% hydroxyethyl starch solution and 6% hydroxyethyl starch solution after the induction of anesthesia in patients undergoing elective neurosurgical procedures. Clinics (Sao Paulo). 2013. 68: 323-8
24. Singla A, Mathew PJ, Jangra K, Gupta SK, Soni SL. A comparison of hypertonic saline and mannitol on intraoperative brain relaxation in patients with raised intracranial pressure during supratentorial tumors resection: A randomized control trial. Neurol India. 2020. 68: 141-5
25. Sokhal N, Rath GP, Chaturvedi A, Singh M, Dash HH. Comparison of 20% mannitol and 3% hypertonic saline on intracranial pressure and systemic hemodynamics. J Clin Neurosci. 2017. 42: 148-54
26. Sorani MD, Morabito D, Rosenthal G, Giacomini KM, Manley GT. Characterizing the dose-response relationship between mannitol and intracranial pressure in traumatic brain injury patients using a high-frequency physiological data collection system. J Neurotrauma. 2008. 25: 291-8
27. Tsaousi GG, Pezikoglou I, Nikopoulou A, Foroglou NG, Poulopoulou A, Vyzantiadis TA. Comparison of equiosmolar doses of 7.5% hypertonic saline and 20% mannitol on cerebral oxygenation status and release of brain injury markers during supratentorial craniotomy: A randomized controlled trial. J Neurosurg Anesthesiol. 2023. 35: 56-64
28. Wu CT, Chen LC, Kuo CP, Ju DT, Borel CO, Cherng CH. A comparison of 3% hypertonic saline and mannitol for brain relaxation during elective supratentorial brain tumor surgery. Anesth Analg. 2010. 110: 903-7