- Department of Neurosurgery, Aga Khan University, Karachi, Pakistan
- Department of Neurosurgery, Liaquat National Hospital, Karachi, Pakistan
- Department of Neurosurgery, Shaukat Khanum Memorial Cancer Hospital and Research Center, Lahore, Pakistan
- Department of Surgery, Section of Dental Surgery, Aga Khan University, Karachi, Pakistan
- Medical School, Liaquat National Hospital and Medical College (LNMC), Liaquat National University, Karachi, Pakistan.
Correspondence Address:
Saad Akhtar, Department of Neurosurgery, Liaquat National Hospital, Karachi, Pakistan.
DOI:10.25259/SNI_907_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Muhammad Yousuf Ul Islam1, Saad Akhtar2, Roua Nasir1, Saad Bin Anis3, Haissan Iftikhar1, Farhan Raza Khan4, Russell Seth Martins1, Muhammad Ehsan Bari1, Urooba Ahmed5. Comparing redo surgery and stereotactic radiosurgery for recurrent, residual, and/or tumors showing progression in nonfunctioning pituitary adenomas: A systematic review and meta-analysis. 09-Feb-2024;15:37
How to cite this URL: Muhammad Yousuf Ul Islam1, Saad Akhtar2, Roua Nasir1, Saad Bin Anis3, Haissan Iftikhar1, Farhan Raza Khan4, Russell Seth Martins1, Muhammad Ehsan Bari1, Urooba Ahmed5. Comparing redo surgery and stereotactic radiosurgery for recurrent, residual, and/or tumors showing progression in nonfunctioning pituitary adenomas: A systematic review and meta-analysis. 09-Feb-2024;15:37. Available from: https://surgicalneurologyint.com/surgicalint-articles/12738/
Abstract
Background: Non-functioning pituitary adenomas (NFPAs) are well-differentiated benign tumors originating from the adenohypophyseal cells of the pituitary gland. They present with headaches, visual disorders, or cranial nerve deficits. NFPAs can recur, progress, or present as residual tumors. We, therefore, conducted this review to compare the effects of both revision surgery and stereotactic surgery on tumor size, visual status, endocrine status, and complications.
Methods: A systematic review of published literature on recurrent, residual, or progressing NFPAs that underwent redo surgery or stereotactic radiosurgery from the inception till June 2020 was conducted as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Thirteen records (1209 patients) were included, and risk ratio (RR) and 95% confidence intervals (CIs) estimated from each study were pooled using a random-effects meta-analysis model.
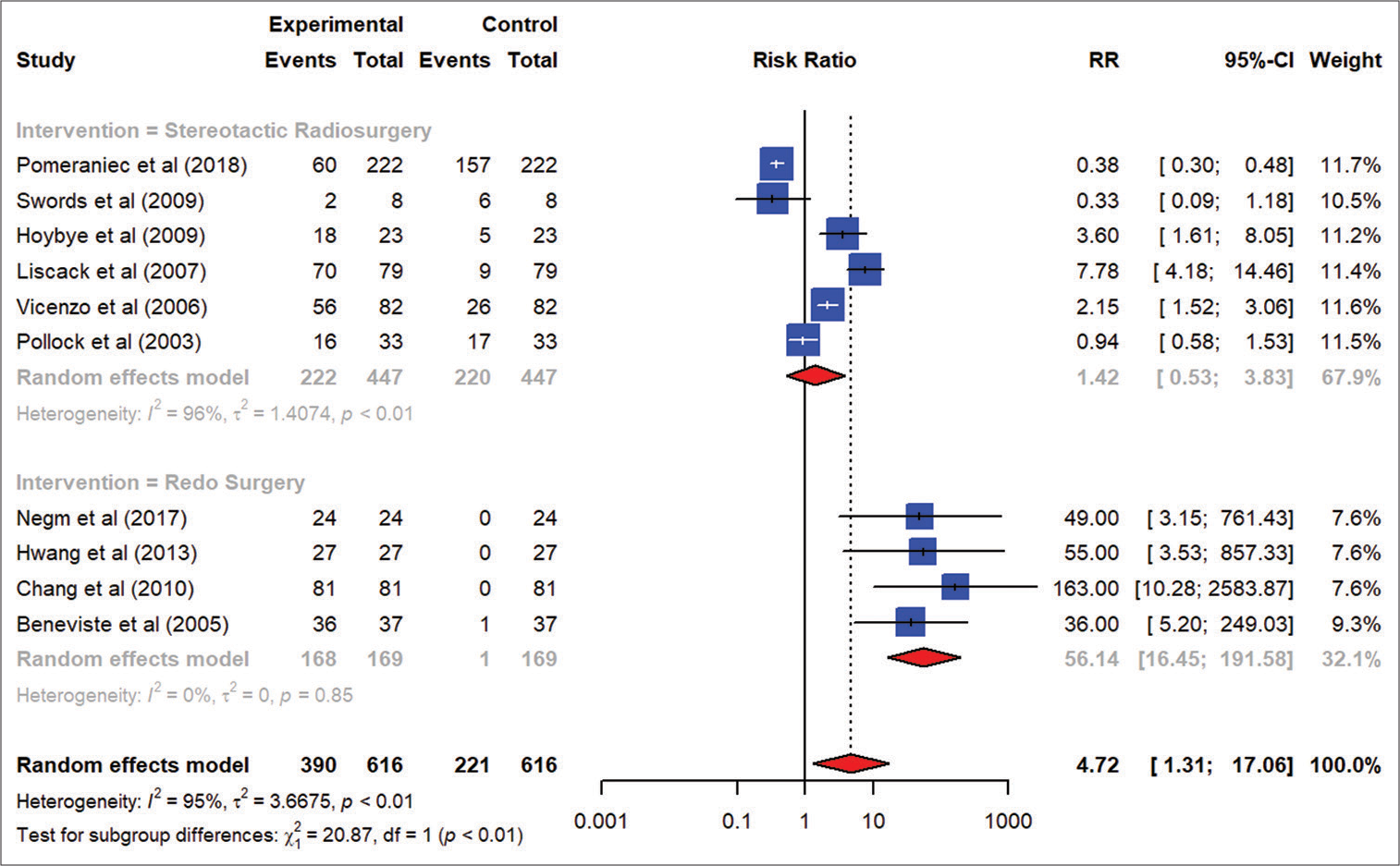
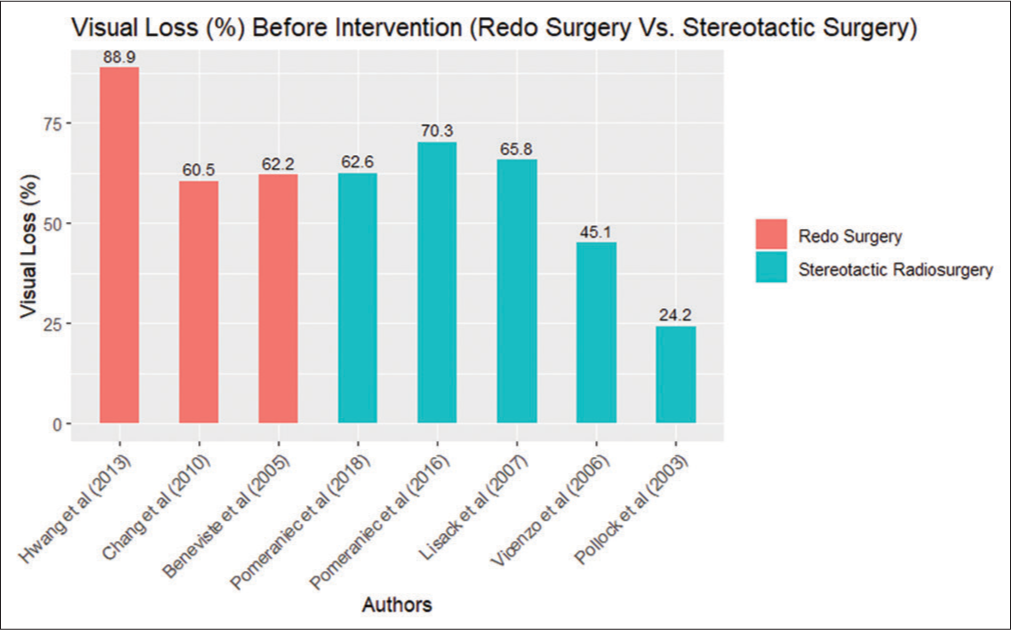
Results: Redo surgery was the preferred intervention in patients presenting with larger tumor sizes and was more effective in reducing the tumor size as compared to stereotactic radiosurgery (SRS) (risk ratio [RR] 56.14; 95% CI, 16.45–191.58). There was more visual loss with revision surgery as compared to SRS (risk ratio [RR] 0.08; 95% CI, 0.03–0.20). However, SRS was associated with fewer complications, such as new diabetes insipidus, as compared to the redo surgery (risk ratio [RR] 0.01; 95% CI 0.01–0.03).
Conclusion: Redo surgery is the superior choice in the treatment of recurrent/residual or progressing NFPAs if the tumor size is large and an immediate reduction in tumor burden through debulking is warranted. However, redo surgery is associated with a higher risk of visual loss, new endocrinopathies, and other complications, in contrast to SRS.
Keywords: Endocrinopathy, NFPA, Nonfunctioning pituitary adenoma, Non-functioning pituitary tumors, PitNets, Stereotactic surgery, Transsphenoidal approach, Transsphenoidal surgery
INTRODUCTION
Non-functioning pituitary adenomas (NFPAs) are well-differentiated benign tumors that originate from the adenohypophyseal cells of the pituitary gland and do not present with symptoms of hormonal hypersecretion.[
The treatment options for NFPAs include observation with serial neuroimaging, repeat surgical intervention through either the transsphenoidal (microscopic or endoscopic) or transcranial route, stereotactic radiosurgery (SRS), fractionated or hypofractionated radiotherapy, and systemic medical therapy (cabergoline or temozolomide). However, NFPAs can recur, undergo progression, or present as residual tumors after incomplete resection and, thus, can be treated after primary surgery by either a redo surgery or SRS.
Surgical resection is the primary treatment for symptomatic patients with NFPAs [
Objective
The objective of this systematic review was to compare the effect of redo surgery and stereotactic surgery in terms of reduction of tumor size, deterioration of vision and existing hypopituitarism, and development of any new endocrinopathies or complications.
MATERIALS AND METHODS
Selection criteria
Inclusion criteria
We included studies published in the English language and with designs ranging from randomized controlled trials (RCTs) and quasi-experimental studies to cohort and cross-sectional studies that reported recurrent or residual disease and/or tumors with progression in NFPAs for which either transsphenoidal or transcranial surgical resection or SRS was performed. No restrictions on publication date were put. However, no RCTs were found on this topic.
Exclusion criteria
We excluded non-English publications, as well as qualitative studies like case reports or case series and reports that involved participants without a diagnosis of NFPAs.
Study selection
This review included observational cohort studies as well as cross-sectional studies.
Study participants
Our review included patients diagnosed with NFPAs.
Types of interventions
We included records that involved either endoscopic or microscopic transsphenoidal surgical resection or transcranial surgical resection or SRS for the treatment of NFPAs.
Outcomes measures
The primary outcomes were the proportion of patients with a reduction in tumor size, further deterioration in hypopituitarism, and worsening of vision after either redo surgery or SRS. Secondary outcomes were any new complications or endocrinopathies. Changes in the vision were defined as any subjective or objective improvement or deterioration in the visual fields after the intervention, as compared to the preintervention examination. Since there was massive heterogeneity in the way vision was tested, we included both subjective and objective measurements. Likewise, changes in the pituitary gland’s function were defined as any improvement or deterioration in the pituitary gland’s secretory function after the intervention, as compared to the preintervention status (based on hormone replacement requirement). Similarly, changes in the adenoma size were defined as at least a 15% increase or decrease in the size of the tumor as appreciated on a magnetic resonance imaging (MRI) scan conducted at least six months or more after the intervention.
Search strategy
The literature search was conducted systematically across multiple databases, including PubMed, Embase, Web of Science, and Wiley Cochrane Library, spanning from 1970 to June 2020. Various permutations of MESH terms “Stereotactic Radiosurgery” OR “GammaKnife Surgery” OR “CyberKnife Surgery” OR “Radiotherapy” OR “Stereotactic Radiotherapy” OR “Hypofractionated Radiotherapy” OR “Fractionated Radiotherapy” OR “Redo Surgery” OR “Repeat Surgery” OR “Revision Surgery” OR “Repeat Transsphenoidal Surgery” OR “Transcranial Surgery” OR “Endoscopic Endonasal Surgery” OR “Microscopic Transsphenoidal Surgery” OR “Endoscopic Transsphenoidal Surgery” AND “Residual Nonfunctioning Pituitary Adenomas” OR “Recurrent Nonfunctioning Pituitary Adenomas” OR “Silent Pituitary Adenomas” OR “NFPAs” were applied. After applying the rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology [
Data collection and analysis
Selection of studies
Initial screening was done independently by two reviewers based on the inclusion criteria. Those records that did not align with the scope of our systematic review and meta-analysis were excluded. Full-text articles were selected as eligible studies and assessed for definitive inclusion as part of secondary screening by two independent reviewers as per the intervention arms and types of outcome measures. A third reviewer addressed any conflicts.
Data extraction and analysis
The data were extracted on a predefined template in Microsoft Excel with variables related to study design, patient demographics, tumor characteristics, and clinical outcomes. The data were subsequently analyzed utilizing both Microsoft Excel and R software to generate forest and funnel plots.
Assessment of methodological quality
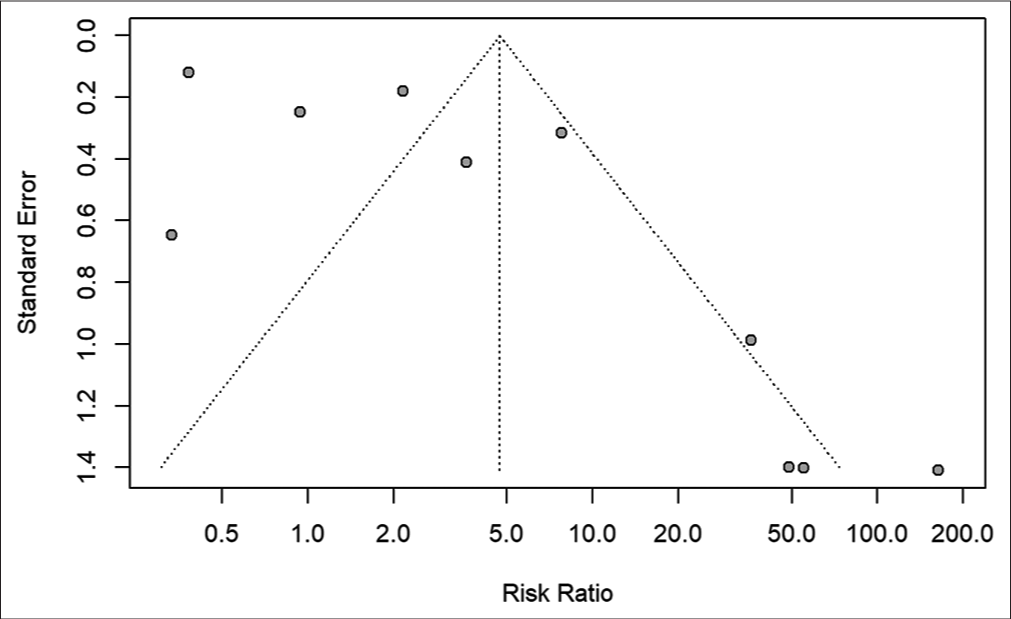
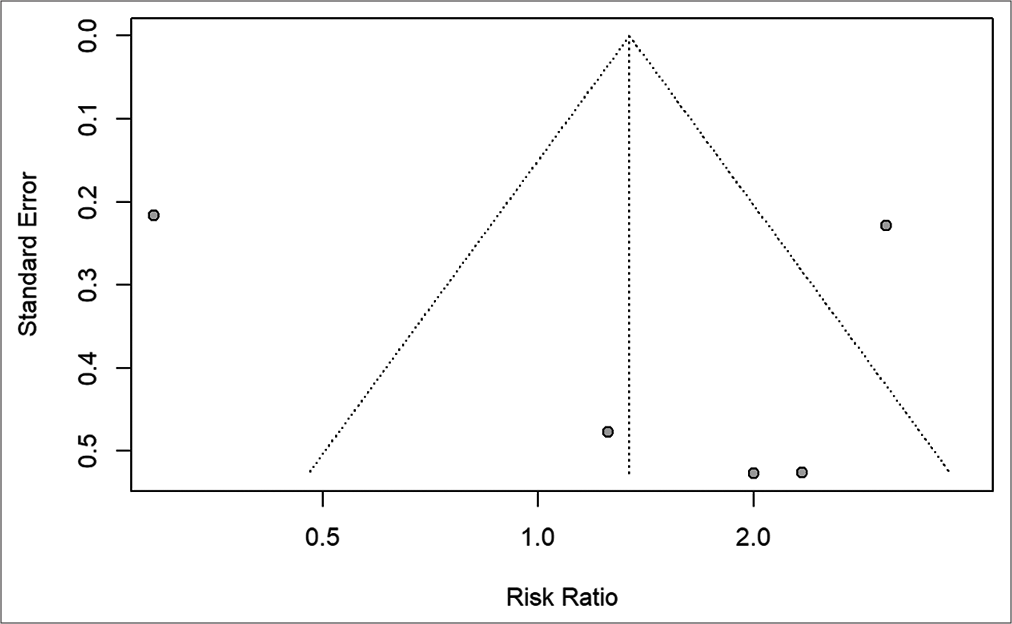
The filtered articles were graded using a modified NIH risk of bias tool for observational cohort and cross-sectional studies to assess the methodological quality and possible bias within the study designs. Funnel plots are displayed according to the publishing bias.
RESULTS
Study selection process
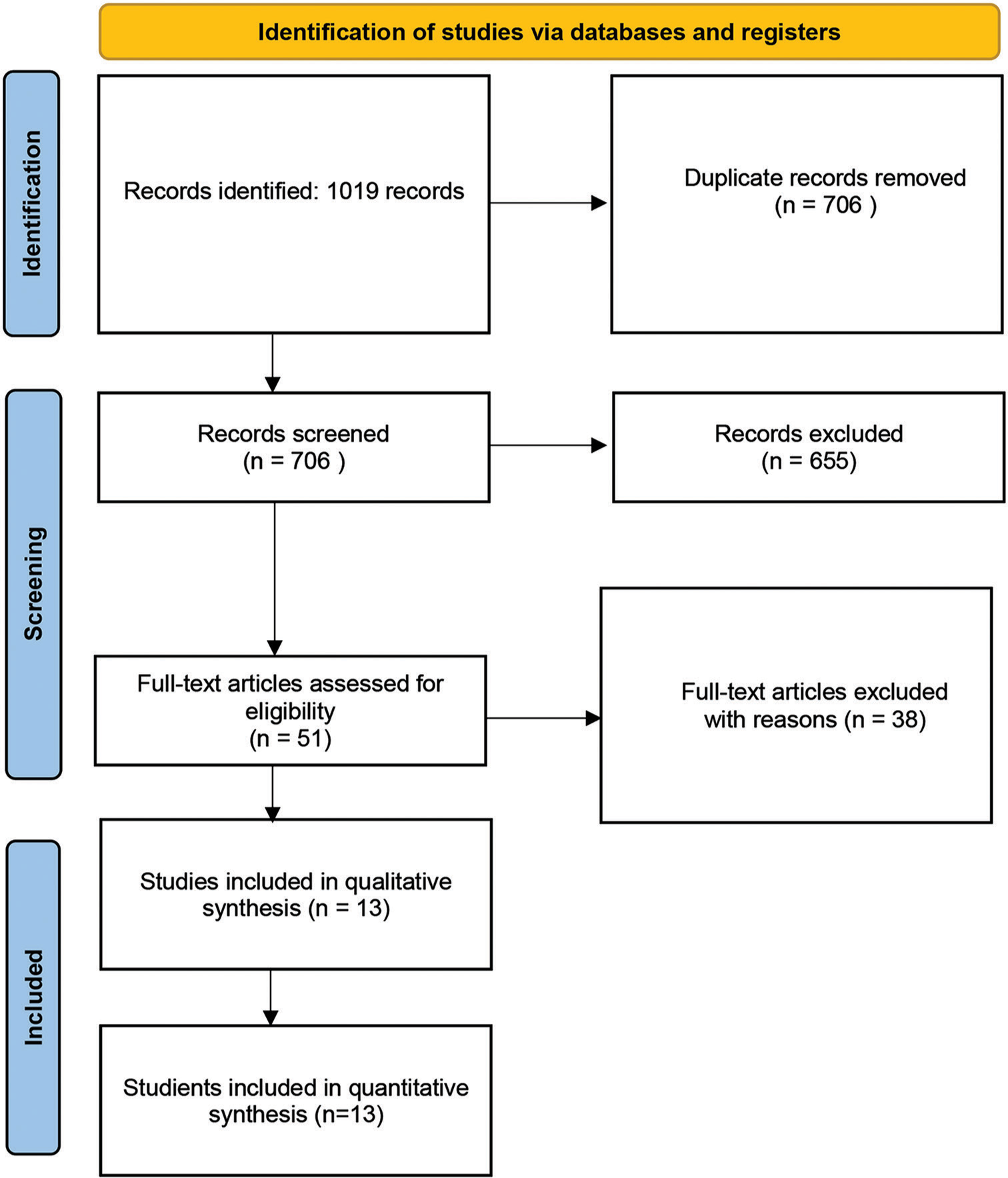
The PRISMA flowchart is given in
Critical appraisal
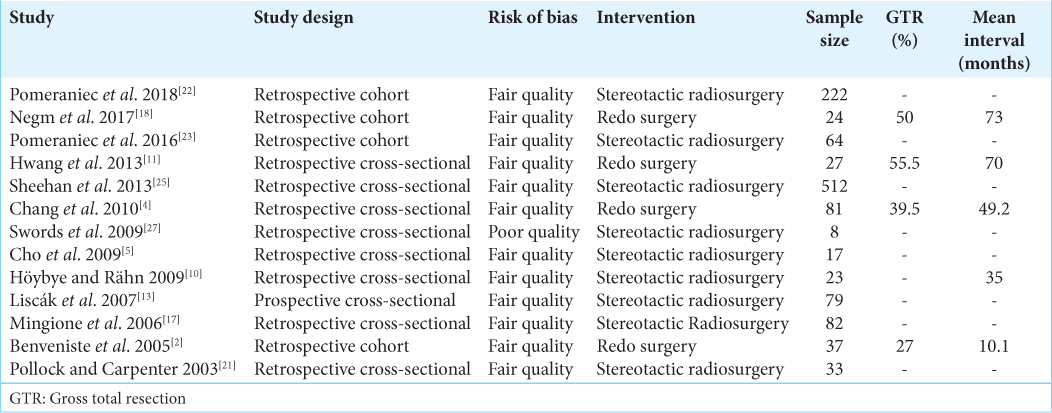
According to the assessment by the modified NIH risk of bias tool for observational cohort and cross-sectional studies, all of our studies were graded “fair quality,” but one was of “poor quality.” The assessment is given in
Redo surgery
Not much information was available on the type of initial surgery in redo surgery, and when redo surgery was performed, it could not be ascertained from the records whether it was microscopic or endoscopic—however, studies by Hwang et al. and Nejm et al.[
Benveniste et al.[
Chang et al.[
Hwang et al.[
Negm et al.[
SRS
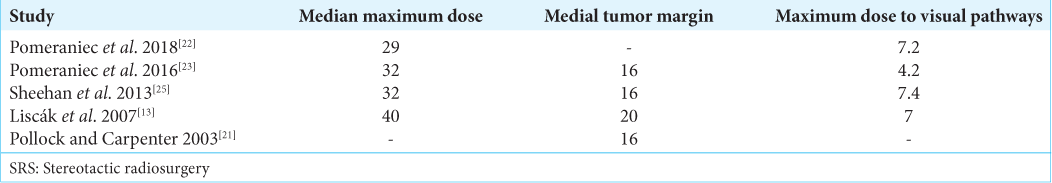
From the records with complete information, the median maximum dose of SRS provided ranged from 29 Gy to 40 Gy, with the median tumor margin dose ranging from 16 Gy to 20 Gy, and the maximum dose to visual pathways staying in a close range of 7–7.4 Gy. The details of SRS are elucidated in
Pollock and Carpenter[
Mingione et al.[
Liscák et al.,[
Cho et al.[
Sheehan et al.[
Pomeraniec et al.[
Pomeraniec et al.[
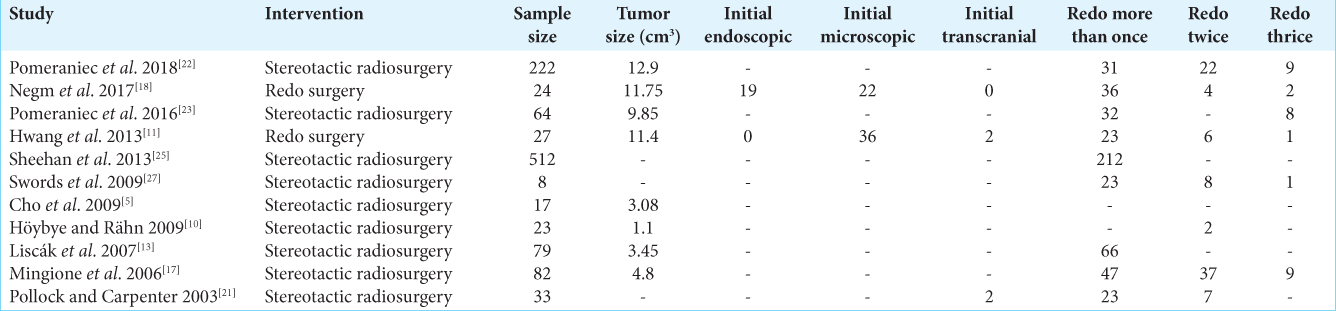
Tumor size
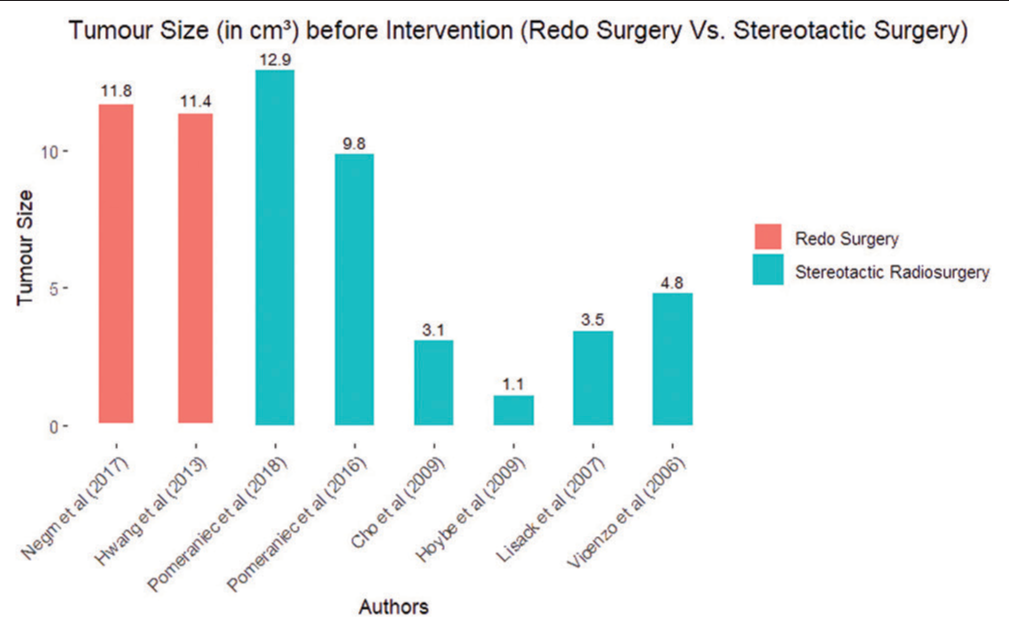
Revision surgery was more commonly done in the cohort of patients presenting with larger tumor sizes, as shown in
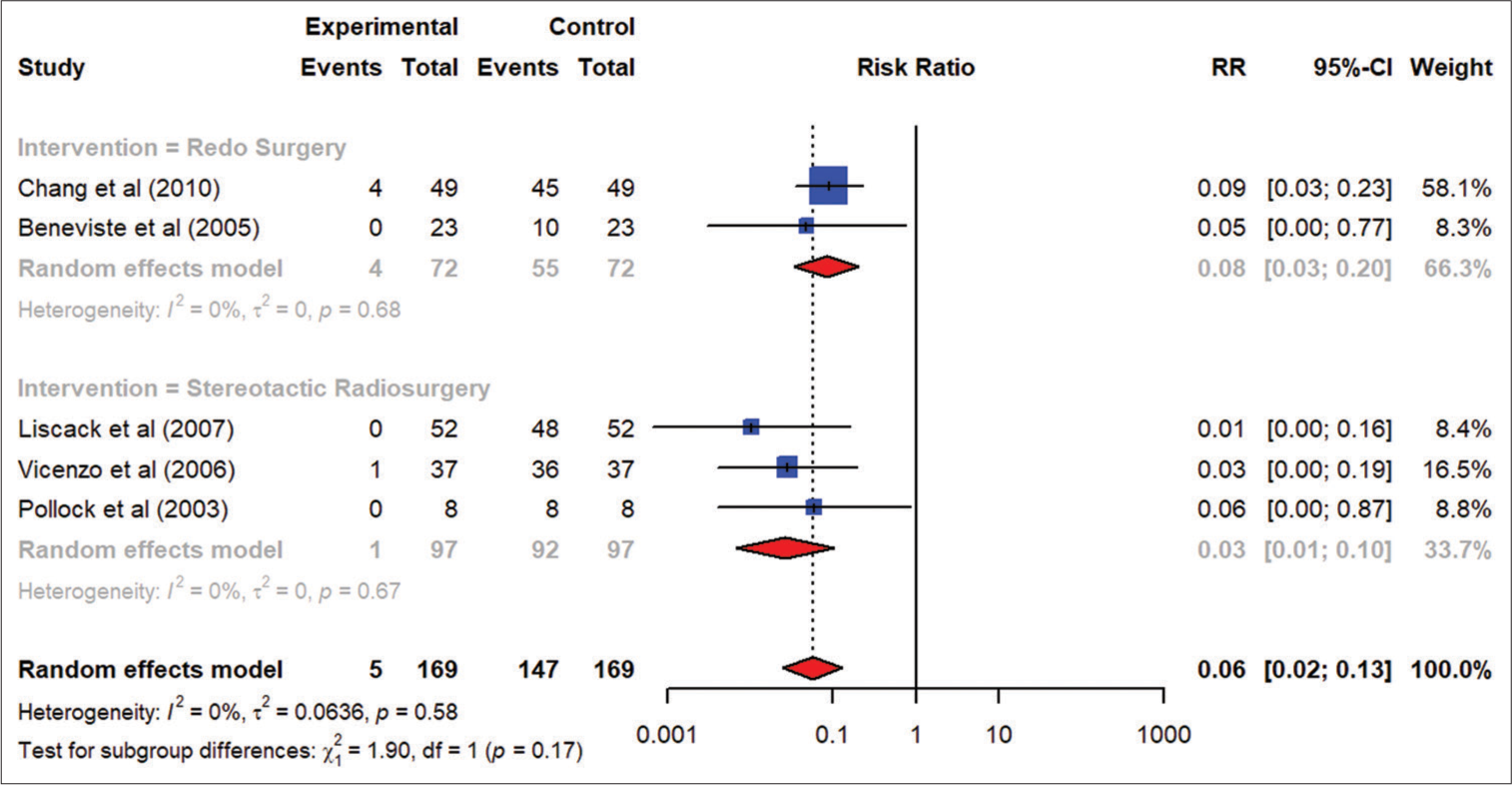
Visual loss
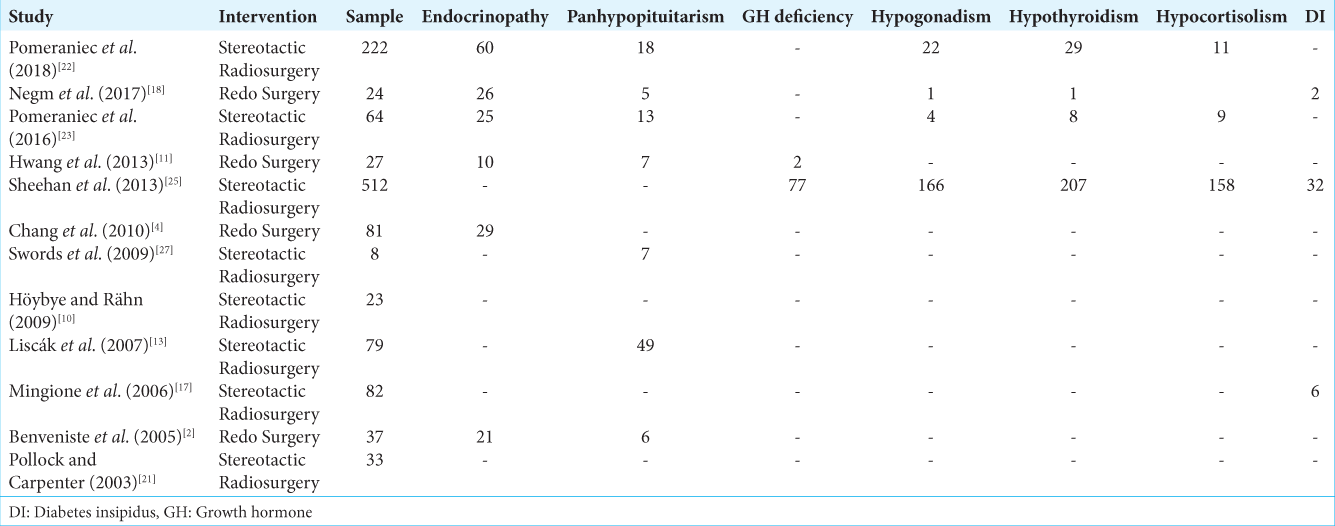
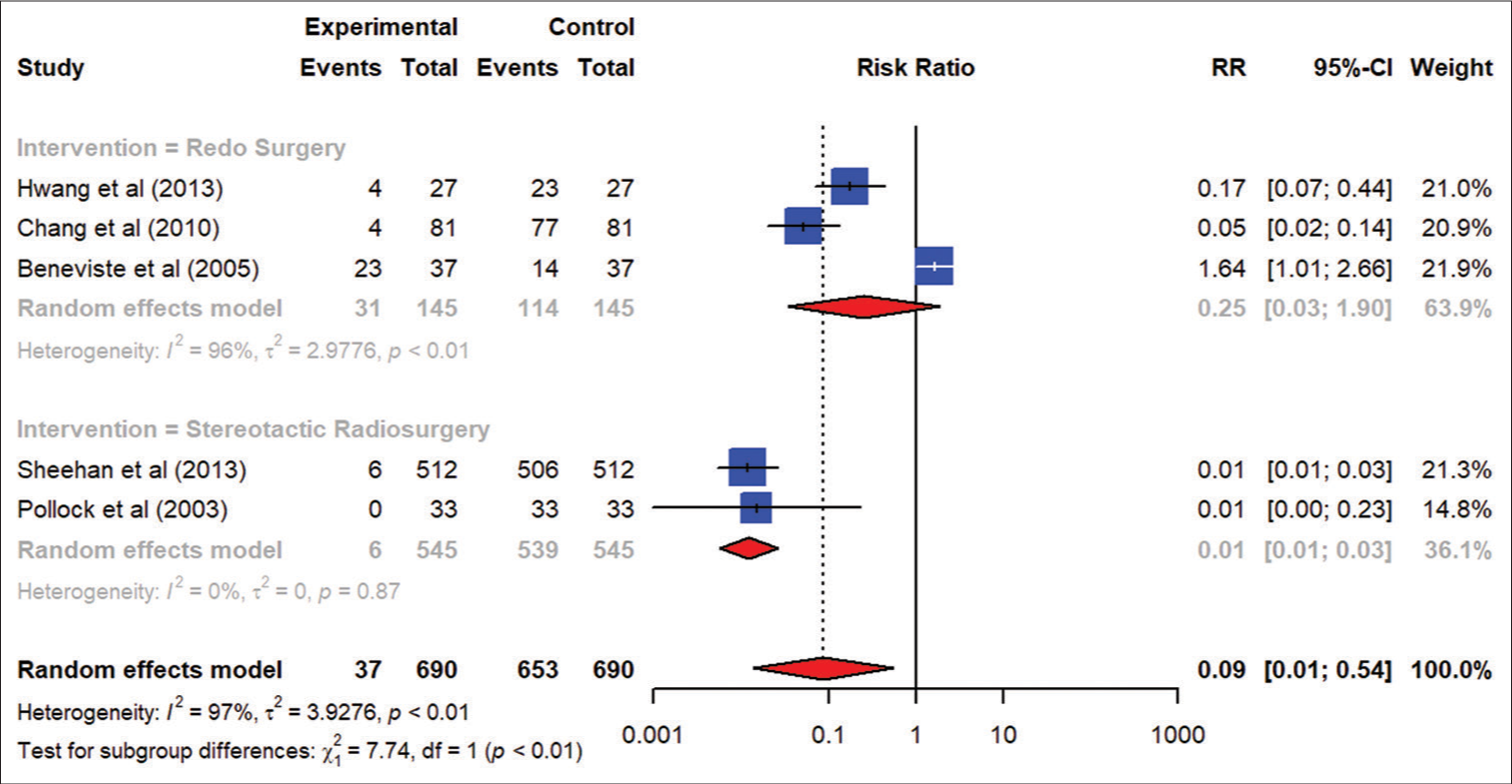
Endocrinopathies
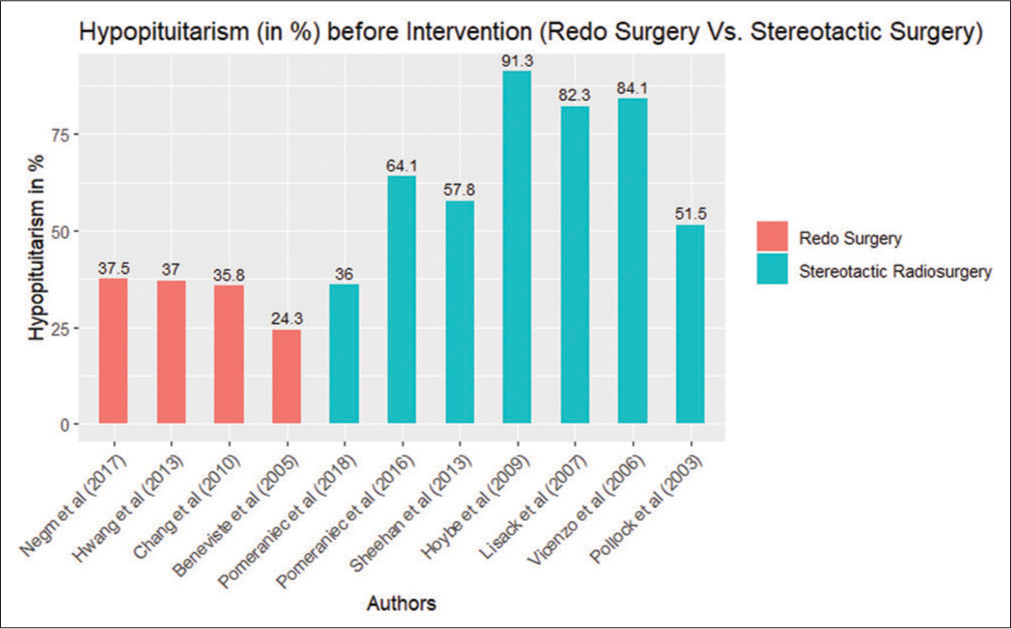
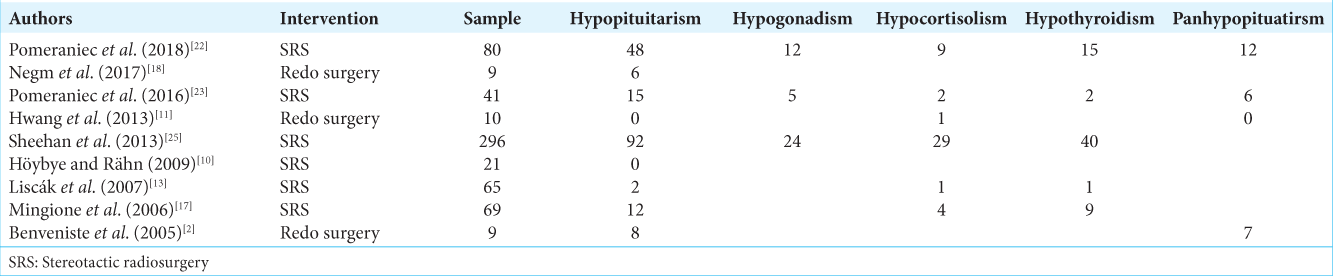
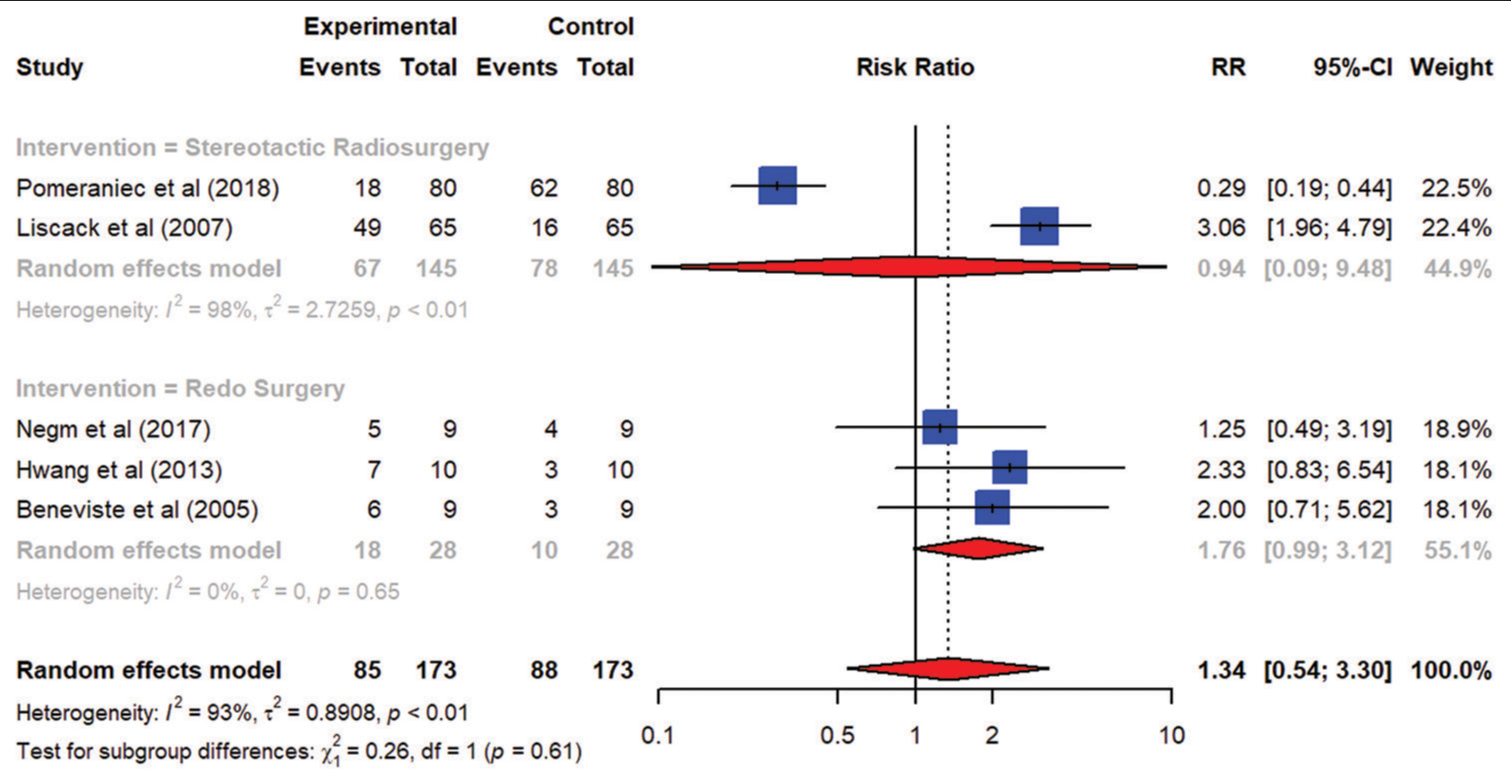
Our results indicate that hypopituitarism was more prevalent in the intervention arm of SRS as compared to revision surgery in
As
DISCUSSION
To the best of our review, no systematic review or a meta-analysis exists that compares the two major intervention arms of redo/revision surgery and stereotactic radiosurgery/radiotherapy in recurrent, residual or tumors with progression in NFPAs. The probability of tumor growth is estimated to be 7.8% and 14.5% at 3 and 5 years without any intervention in the form of surgery or radiotherapy.[
Hypopituitarism is defined as a deficiency of one or more pituitary hormones. Patients with NFPAs develop hypopituitarism due to mechanical compressive forces generated on the pituitary gland with the consequent effects on the gland’s portal circulation. Hypopituitarism is a relative indication of surgery in patients with NFPAs and is found more commonly in patients who are symptomatic, of male gender, and those harboring macroadenomas.[
Revision surgery results in a more exhaustive tumor mass removal due to the physical nature of removal, leading to the prompt alleviation of pressure exerted on adjacent anatomical structures as compared to stereotactic radiosurgery (SRS). The transsphenoidal surgical approach is proven to be safe and effective but is unable to remove tumors fully; thus, a combination of transcranial and transsphenoidal approaches (TSAs) can be utilized to maximize tumor removal. The microscopic and endoscopic TSAs are similar in their safety profiles; however, endoscopic is the superior modality in terms of preserving the postoperative hormonal profile.[
SRS, however, precisely and non-invasively delivers targeted radiation therapy to the tumor site to shrink or control the tumor and judiciously preserves surrounding healthy tissue. This makes SRS an inherently less invasive therapeutic option uniquely tailored to smaller intracranial lesions, and thus not suitable for large or complex tumors. It is a viable option when surgery is not advisable, especially in frail and elderly populations. It is designed to minimize damage to surrounding healthy tissue, including the optic nerves and pituitary gland. In our review, besides Cho et al.[
The choice between redo surgery and SRS should be made on a case-by-case basis, taking into consideration the specific characteristics of the tumor (such as initial tumor size) and the condition of the patient. Patients must discuss their treatment options with their medical team to determine the most appropriate approach.
Limitations
There is a notable scarcity of prospective studies and clinical trials available to offer definitive guidance for treatment decisions in cases of recurrent pituitary adenomas. Our study encountered a significant limitation in the form of a lack of class I articles that would have allowed for direct comparisons with our study models. Moreover, the new records from 2021 to 2023 were not included in our systematic review. Furthermore, our SRS studies were constrained by a follow-up period averaging <5 years. Typically, SRS requires approximately 5–10 years to manifest its complete effects. The inherent delay in the response to SRS poses a logistical challenge when considering the feasibility of conducting a RCT for an effective comparison of these two treatment modalities. This delay may, in part, contribute to the potential underestimation of the outcomes associated with SRS in our review. We did not include the comparison based on histopathological subtypes or immunohistochemical analysis. In addition, there was no long-term data available to address radiation-induced neoplasms, rates of tumor recurrence, and development of new endocrinopathies.
CONCLUSION
Redo surgery is the superior choice in the treatment of recurrent/residual tumors showing progression in diagnosed NFPA cases if the tumor size is large and an immediate reduction in tumor burden through debulking is warranted. However, redo surgery is associated with a higher risk of visual loss, new endocrinopathies, and other complications, in contrast to SRS. SRS, with a conformal regimen that spares the pituitary gland and optic apparatus, can be a viable alternative in tumors of small size, frail or elderly populations, and can achieve a satisfactory tumor control as well as avoid complications.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s was consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Banskota S, Adamson DC. Pituitary adenomas: From diagnosis to therapeutics. Biomedicines. 2021. 9: 494
2. Benveniste RJ, King WA, Walsh J, Lee JS, Delman BN, Post KD. Repeated transsphenoidal surgery to treat recurrent or residual pituitary adenoma. J Neurosurg. 2005. 1026: 1004-12
3. Česák T, Póczoš P, Adamkov J, Čelakovský P, Gabalec F, Soukup J. Microsurgical versus endoscopic surgery for nonfunctioning pituitary adenomas: A retrospective study. Croat Med J. 2020. 61: 410-21
4. Chang EF, Sughrue ME, Zada G, Wilson CB, Blevins LS, Kunwar S. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary. 2010. 13: 223-9
5. Cho CB, Park HK, Joo WI, Chough CK, Lee KJ, Rha HK. Stereotactic radiosurgery with the cyberknife for pituitary adenomas. J Korean Neurosurg Soc. 2009. 45: 157-63
6. Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010. 72: 377-82
7. Guerreiro V, Mendonça F, Ferreira HU, Freitas P, Pereira J, Bernardes I. Incidental versus symptomatic nonfunctioning pituitary adenomas: Are they different?. Endocrinol Diabetes Metab. 2023. 6: e445
8. Hamblin R, Fountas A, Lithgow K, Loughrey PB, Bonanos E, Shinwari SK. Natural history of nonfunctioning pituitary microadenomas: Results from the UK nonfunctioning pituitary adenoma consortium. Eur J Endocrinol. 2023. 189: 87-95
9. Horvath E, Kovacs K, Killinger DW, Smyth HS, Platts ME, Singer W. Silent corticotropic adenomas of the human pituitary gland: A histologic, immunocytologic, and ultrastructural study. Am J Pathol. 1980. 98: 617-38
10. Höybye C, Rähn T. Adjuvant Gamma Knife radiosurgery in nonfunctioning pituitary adenomas; low risk of long-term complications in selected patients. Pituitary. 2009. 12: 211-6
11. Hwang JM, Kim YH, Kim JW, Kim DG, Jung HW, Chung YS. Feasibility of endoscopic endonasal approach for recurrent pituitary adenomas after microscopic trans-sphenoidal approach. J Korean Neurosurg Soc. 2013. 54: 317-22
12. Kinoshita Y, Kurisu K, Arita K. Nonfunctioning pituitary adenomas in elderly patients. J Clin Neurosci. 2018. 53: 127-31
13. Liscák R, Vladyka V, Marek J, Simonová G, Vymazal J. Gamma knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir (Wien). 2007. 149: 999-1006 discussion 1006
14. Lucas JW, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, Litvack Z. Congress of neurological surgeons systematic review and evidence-based guideline on primary management of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016. 79: E533-5
15. Mattozo CA, Dusick JR, Esposito F, Mora H, Cohan P, Malkasian D. Suboptimal sphenoid and sellar exposure: A consistent finding in patients treated with repeat transsphenoidal surgery for residual endocrine-inactive macroadenomas. Neurosurgery. 2006. 58: 857-65
16. Mavromati M, Mavrakanas T, Jornayvaz FR, Schaller K, Fitsiori A, Vargas MI. The impact of transsphenoidal surgery on pituitary function in patients with nonfunctioning macroadenomas. Endocrine. 2023. 81: 340-8
17. Mingione V, Yen CP, Vance ML, Steiner M, Sheehan J, Laws ER. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg. 2006. 104: 876-83
18. Negm HM, Al-Mahfoudh R, Pai M, Singh H, Cohen S, Dhandapani S. Reoperative endoscopic endonasal surgery for residual or recurrent pituitary adenomas. J Neurosurg. 2017. 127: 397-408
19. Ntali G, Wass JA. Epidemiology, clinical presentation and diagnosis of nonfunctioning pituitary adenomas. Pituitary. 2018. 21: 111-8
20. Øystese KA, Evang JA, Bollerslev J. Nonfunctioning pituitary adenomas: Growth and aggressiveness. Endocrine. 2016. 53: 28-34
21. Pollock BE, Carpenter PC. Stereotactic radiosurgery as an alternative to fractionated radiotherapy for patients with recurrent or residual nonfunctioning pituitary adenomas. Neurosurgery. 2003. 53: 1086-91 discussion 1091-1094
22. Pomeraniec IJ, Kano H, Xu Z, Nguyen B, Siddiqui ZA, Silva D. Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: A multicenter matched-cohort study. J Neurosurg. 2018. 129: 648-57
23. Pomeraniec IJ, Dallapiazza RF, Xu Z, Jane JA, Sheehan JP. Early versus late Gamma Knife radiosurgery following transsphenoidal resection for nonfunctioning pituitary macroadenomas: A matched cohort study. J Neurosurg. 2016. 125: 202-12
24. Reddy R, Cudlip S, Byrne JV, Karavitaki N, Wass JA. Can we ever stop imaging in surgically treated and radiotherapy-naive patients with nonfunctioning pituitary adenoma?. Eur J Endocrinol. 2011. 165: 739-44
25. Sheehan JP, Starke RM, Mathieu D, Young B, Sneed PK, Chiang VL. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: A multicenter study. J Neurosurg. 2013. 119: 446-56
26. Sunil A, Thakar S, Aryan S, Hegde AS. Changes in sinonasal and overall quality of life following endoscopic endonasal surgery for nonfunctioning pituitary adenomas: Results of a prospective observational study. Neurol India. 2022. 70: 2357-65
27. Swords FM, Monson JP, Besser GM, Chew SL, Drake WM, Grossman AB. Gamma knife radiosurgery: A safe and effective salvage treatment for pituitary tumours not controlled despite conventional radiotherapy. Eur J Endocrinol. 2009. 161: 819-28
28. Tjörnstrand A, Gunnarsson K, Evert M, Holmberg E, Ragnarsson O, Rosén T. The incidence rate of pituitary adenomas in western Sweden for the period 2001-2011. Eur J Endocrinol. 2014. 171: 519-26
29. Yamamoto M, Aiyama H, Koiso T, Watanabe S, Kawabe T, Sato Y. Postsurgical salvage radiosurgery for nonfunctioning pituitary adenomas touching/compressing the optic chiasm: Median 13-year post-irradiation imaging follow-up results. Neurosurgery. 2019. 85: 476-85
30. Zhang Z, Wang P, Feng S, Yu X. Endocrinological outcomes of intraoperative MRI-guided endoscopic transsphenoidal surgery for nonfunctioning pituitary adenoma. Turk Neurosurg. 2019. 29: 635-42