- Department of Anaesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- Department of Biochemistry and Pharmacology, Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, Parkville, Australia,
- Department of Neuroanaesthesia and Neurocritical Care, Artemis Hospitals, Gurugram, Haryana, India
- Department of Anesthesia and Intensive Care, Dr Rajendra Prasad Goverment Medical College, Kangra, Himachal Pradesh, India
- Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- Department of Nursing, National Institute of Nursing Education, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Hemant Bhagat, Department of Anaesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_788_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shalvi Mahajan1, Tanavi Sharma2, Nidhi Bidyut Panda1, Rajeev Chauhan1, Steve Joys3, Nanish Sharma4, Manju Mohanty5, Navneet Singla5, Sanjay Kumar1, Ashok Kumar6, Hemant Bhagat1. Comparison of propofol and desflurane for postoperative neurocognitive function in patients with aneurysmal subarachnoid hemorrhage: A prospective randomized trial. 15-Mar-2024;15:84
How to cite this URL: Shalvi Mahajan1, Tanavi Sharma2, Nidhi Bidyut Panda1, Rajeev Chauhan1, Steve Joys3, Nanish Sharma4, Manju Mohanty5, Navneet Singla5, Sanjay Kumar1, Ashok Kumar6, Hemant Bhagat1. Comparison of propofol and desflurane for postoperative neurocognitive function in patients with aneurysmal subarachnoid hemorrhage: A prospective randomized trial. 15-Mar-2024;15:84. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12804
Abstract
Background: Following aneurysmal subarachnoid hemorrhage, 40–50% of survivors experience cognitive dysfunction, which affects their quality of life. Anesthetic agents play a pivotal role in aneurysm surgeries. However, substantial evidence regarding their effects on neurocognitive function is lacking. This study evaluated the effects of propofol and desflurane on postoperative neurocognitive function and serum S-100B levels.
Methods: One hundred patients were equally randomized to receive either propofol (Group P) or desflurane (Group D). Cognitive function was assessed using the Montreal Cognitive Assessment scale at three different time points: Preoperatively, at the time of discharge, and one month after surgery. Perioperative serum levels of S-100B were also measured.
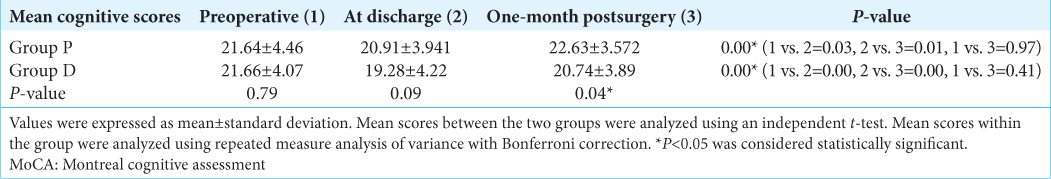
Results: The preoperative mean cognitive score in Group P was 21.64 + 4.46 and in Group D was 21.66 + 4.07 (P = 0.79). At discharge, a significant decrease in cognitive scores was observed compared to preoperative scores (Group P- 20.91 + 3.94, P = 0.03 and Group D-19.28 + 4.22, P = 0.00); however, scores were comparable between the two groups (P = 0.09). One month following surgery, mean cognitive scores were 22.63 + 3.57 in Group P and 20.74 + 3.89 in Group D, and the difference was significant (P = 0.04). Higher memory and orientation scores were observed in Group P than in Group D at one month (P
Conclusion: The mean cognitive scores one month after surgery improved significantly with propofol compared with desflurane, but without clinical significance. Individual domain analysis demonstrated that orientation and memory scores were better preserved with propofol.
Keywords: Aneurysmal subarachnoid hemorrhage, Desflurane, Montreal cognitive assessment test, Postoperative cognitive function, Propofol, S-100B
INTRODUCTION
Spontaneous rupture of intracranial aneurysm leads to aneurysmal subarachnoid hemorrhage (aSAH). Affected patients develop a neurocognitive deficit in the postoperative period leading to the inability to restitute their daily routine.[
Anesthetic agents may affect a patient’s cognitive function postoperatively due to their inherent action at multiple sites in the brain, which may alter neuronal activity. There is a dearth of literature on the effect of anesthetic agents on neurocognitive functions in such patients. Propofol is a widely preferred agent in neurosurgery because it decreases the cerebral metabolic rate of oxygen consumption (CMRO2) and cerebral blood flow (CBF) and maintains cerebrovascular reactivity to carbon dioxide.[
A pilot trial has shown better cognitive functions with propofol compared to desflurane in aSAH patients at discharge.[
MATERIALS AND METHODS
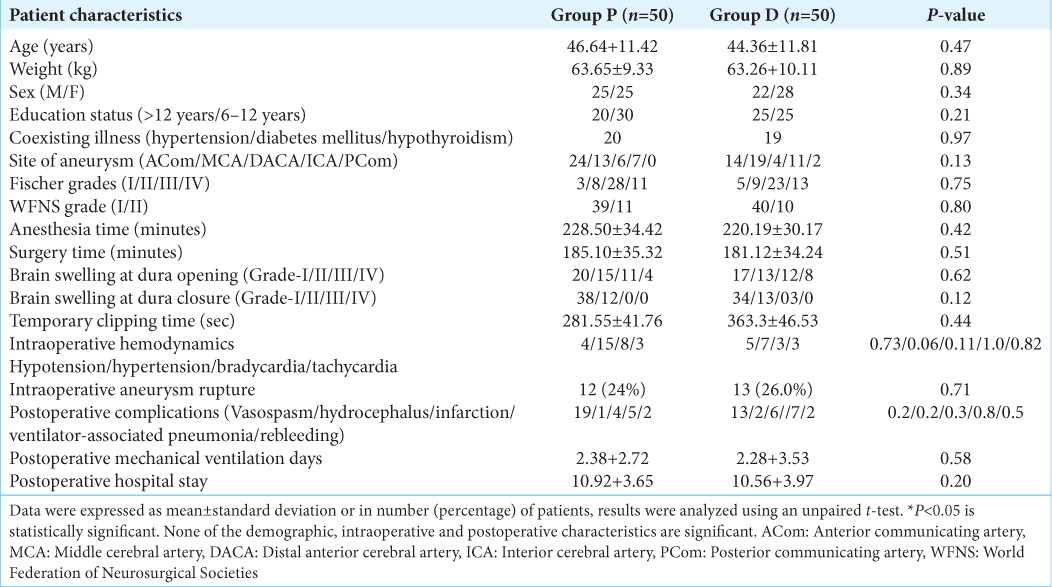
The prospective randomized comparative study was conducted after obtaining approval from the Institutional Ethical Board over 1½ years (INT/IEC/2015/741 dated 19/11/2015). The trial has been registered with a clinical trial government registry (Clinical Trials.gov with a unique identifier: NCT02987218/2016). The written informed consent was obtained from all enrolled patients. The patients scheduled for surgical clipping of aSAH with clinical and radiological evidence of cerebral aneurysm between 18 and 65 years of either sex or World Federation of Neurosurgeon (World Federation of Neurosurgical Societies) grades 1 and 2 were included in the study. Preoperatively, patients with known psychiatric illness, a history of drug abuse, low level of education (illiterate), or multiple failures in school and multiple surgeries were excluded from the study. Intraoperative complications such as massive blood loss, prolonged clipping time (>20 min), severe intraoperative brain swelling precluding replacement of bone flap, and postoperatively, unconscious, intubated, or tracheostomized patients were also excluded from the study.
Randomization and blinding
A total of 100 patients were randomized into Group P and Group D using a computer-generated random number algorithm. These random numbers were kept sequentially in numbered opaque envelopes, and concealment was done by an anesthesiologist who was not involved in the assessment of cognitive functions. The person assessing cognitive functions (clinical psychologist) was blinded to a study drug.
Anesthesia protocol
The standard general anesthesia technique as per institutional protocol was followed. Standard monitors applied were 5-lead electrocardiography, noninvasive automated blood pressure, pulse oximetry, capnography, and entropy. Induction of anesthesia was carried out with propofol in titrated doses. Vecuronium 0.1 mg/kg was used to achieve muscle relaxation. Intraoperative analgesia was achieved with fentanyl two µg/kg bolus followed by 0.5–2 µg/kg/h infusion. Subsequently, as per the randomization, patients were maintained on either propofol or desflurane along with oxygen/air (50/50) and titrated to keep state entropy in the range of 40–60. Mannitol 0.5 g/kg was administered in both groups. Fentanyl infusion was stopped at the beginning of scalp closure whereas the maintenance agents were stopped at the time of application of staples for skin closure. At the end of anesthesia, residual neuromuscular blockade was reversed using neostigmine 50 mcg/kg and glycopyrrolate 10 mcg/kg. Subsequently, patients were extubated, and those who were not extubated were shifted to a neurosurgical intensive care unit for further management.
Intraoperatively, hemodynamic parameters, end-tidal carbon dioxide, and entropy were measured. Intraoperative aneurysm rupture and temporary clipping time were also noted.
The duration of postoperative mechanical ventilation and hospital stays were recorded. Postoperative complications such as vasospasm, hydrocephalus, infarction, ventilator-associated pneumonia, and rebleeding were also noted.
Cognitive function assessment
The cognitive functions were assessed using a Hindi version of the Montreal Cognitive Assessment (MoCA) test, a 30-point scale that includes executive functions, orientation, naming, attention, abstraction, and delayed recall.[
Biomarker S-100B
S-100B levels were measured in serum. Arterial blood samples were collected in plain BD (Becton Dickson) vacutainer. Blood was left undisturbed at room temperature for 30 min. After that, centrifugation of the sample was carried out at 2500 rotation/min for 10 min, and the supernatant was collected. Serum samples were stored at −80° C. Sandwich enzyme-linked immunosorbent assay (ELISA) was performed using precoated ELISA KIT YH Bio search (Cat No-YHB3336Hu) for S-100B (Human). Serum S100B levels were measured preoperatively, intraoperatively after clipping of the neck of the aneurysm, and one h postoperatively.
Sample size calculation
The sample size was estimated based on the mean difference of 3.7 in POCD scores between the propofol group when compared to the desflurane group with a standard deviation (SD) of 5.[
Statistical analysis
Normality of quantitative data was checked using measures of Kolmogorov–Smirnov test. The variance analysis of repeated measurement data was applied for statistical treatment. All measured data were expressed as mean ± SD. The means have been compared using unpaired t-tests between groups. Paired t-test was used in comparison in each group. Categorical variables were analyzed using the Chi-square test and presented as numbers and percentages. All calculations were two-sided and were performed using the Statistical Package for the Social Sciences Inc., version 22.0 for Windows, Armonk, NY, USA. The “P” value <0.05 indicates that the difference has been statistically significant.
RESULTS
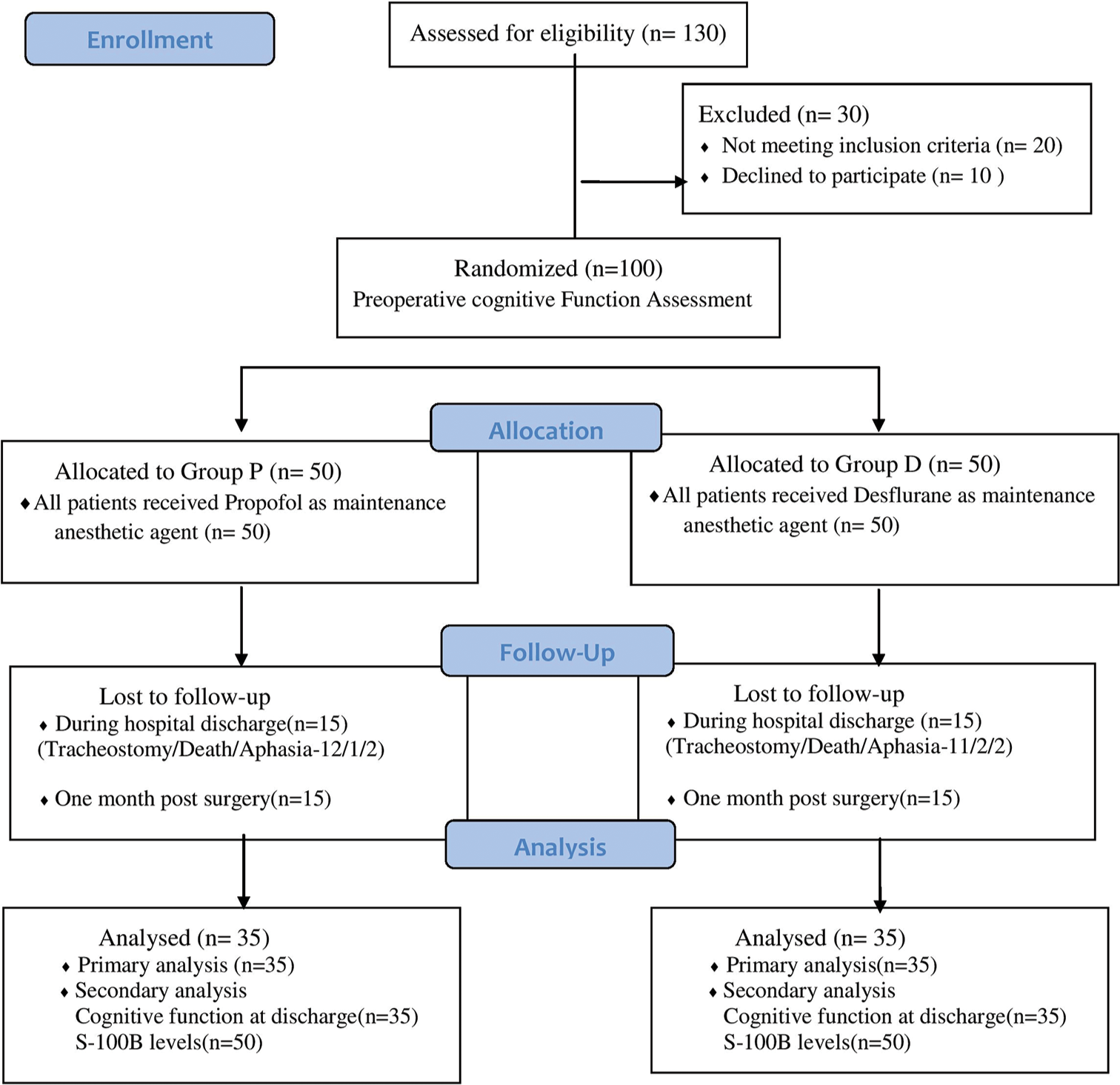
In this study, 100 patients were enrolled and randomized equally into two groups – Group P and Group D. However, data were analyzed for 70 patients (Group P [n = 35], Group D [n = 35]) at 1-month follow-up for the primary outcome of the study [
Cognitive functions
The preoperative cognitive function and that at the discharge from the hospital were similar between Group P and Group D. However, one month following surgery, cognitive functions were significantly better with the use of propofol compared to desflurane [
During preoperative assessment, the mean cognition score in Group P and Group D was not different (P = 0.79). At the time of hospital discharge, the mean cognition score decreased in both groups and was comparable (P = 0.09). One month following surgery, mean scores were higher in Group P (22.63 ± 3.56) compared to Group D (20.74 ± 3.89 ) (P = 0.04) [
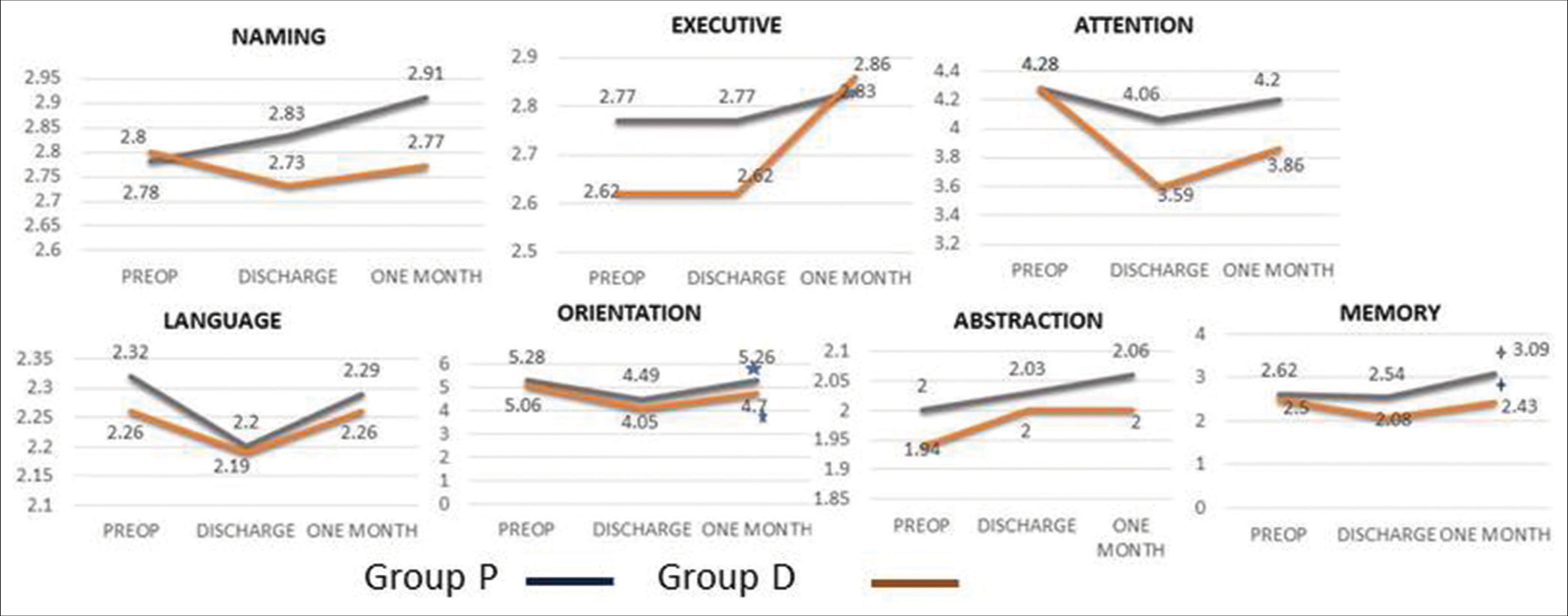
Subgroup analysis of individual domains of cognitive functions within the group (intragroup) revealed no difference in domains such as executive functions, naming, language, and abstraction in both groups [
In intergroup analysis of individual domains of cognitive function, we found no difference in mean cognitive scores during the preoperative period and at the time of discharge. However, one month following surgery, we found significantly higher mean scores in memory and orientation domains of cognition in Group P compared to Group D (P < 0.05) [
Serum S-100B levels (a biomarker of cognitive dysfunction)
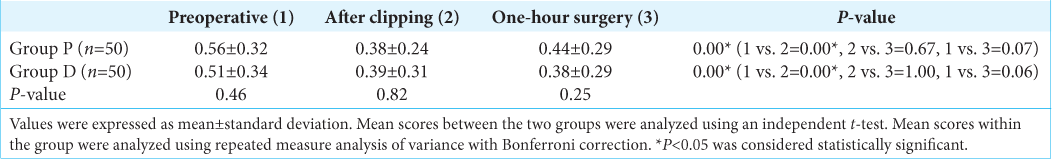
In both groups, intraoperative S-100B levels (measured after clipping of the neck of the aneurysm) were significantly reduced compared to the preoperative value (P = 0.00). However, postoperative values were not significant compared to the preoperative and intraoperative values (P = 0.05) [
DISCUSSION
Both intravenous and inhalational agents have been used for the maintenance of anesthesia during neurosurgery. However, the data comparing various anesthetic agents specifically for cognitive function outcomes following surgery for aneurysmal neck clipping after aSAH is limited. Hence, this prospective randomized control trial analyzed cognitive function with inhalational and intravenous anesthetic agents at one month after craniotomy and clipping in aSAH patients. The study observed that both groups’ preoperative and postoperative discharge scores were comparable. At one month following surgery, propofol was associated with statistically improved cognitive scores compared to desflurane, but clinically there is no improvement with either of the anesthetic agents. When we compared individual domains of cognitive function, most domains were comparable except for memory and orientation, which were better with propofol. The markers of neuroinflammation S-100B were also comparable between the two drug groups.
During aSAH, elevated intracranial pressure (ICP) compromises CBF. When these patients are subjected to anesthesia, exposure to propofol decreases cerebral metabolic rate (CMR) by 48–58%, while desflurane decreases CMR by 35%.[
Using digital subtraction angiography, the effects of propofol and sevoflurane on the cerebral vasculature of patients undergoing endovascular coiling after aSAH were studied.[
It has been shown in a previous study that pharmacological neuroprotection with propofol during temporary clipping did not improve cognitive function in this subset of the population at 24 h postoperatively or during discharge compared with a control group.[
Aneurysmal SAH patients show the most frequent impairment in memory, executive function, and language components.[
Our study observed a decrease in the memory scores with propofol at the time of discharge. However, scores recovered one month after surgery. In the desflurane group, no similar findings were observed. Children exposed to propofol anesthesia have impaired short-term memory at seven days compared to baseline values. However, their memory scores recovered after three months.[
Orientation, a fundamental cognitive function, is mediated by cortical activation in the precuneus, inferior parietal, and medial frontal cortex of the brain. The orientation scores were also found to be decreased at discharge in both the groups, which improved to baseline values at one month following surgery. A stressful perioperative period (stress of surgery, postoperative complications, and lack of home environment) may contribute to low orientation scores at discharge, which may slowly improve over some time.[
In a preliminary trial, we found that propofol preserved executive function, attention, and orientation better than desflurane. In addition, the present study showed that orientation and memory were better maintained with propofol than with desflurane. This could be because a preliminary study with a small sample population was not powered to study endpoints. However, the present study with a larger sample was adequately powered.
A preoperative assessment of cognitive functions is paramount for establishing the patient’s baseline function. However, it is challenging to evaluate them in the preoperative period due to higher reported baseline cognitive dysfunction (70%) in aSAH patients.[
In aSAH, initial mean daily values S-100B levels above 0.4 mcg/L predict a poor outcome in terms of Glasgow outcome score at six months.[
The main strength of our study was that the trial was done prospectively in aSAH patients undergoing craniotomy and clipping. So far, this was the first study in this subset population where cognitive function assessment was done in the preoperative period. Furthermore, biomarker levels were assessed as surrogate markers for cognitive function assessment.
There are a few limitations of this study. First, we assessed cognitive functions using the MoCA test. This test covers six different domains of cognition but does not evaluate individual domains in detail. Hence, a more comprehensive scale might predict the affected domain in a better way and aid in planning the rehabilitation of the patient. Second, the study was done in only a modest number of patients in a single center. Larger multicenter studies are required to substantiate the results before a definitive conclusion is made. Third, we included only good grade patients. However, poor-grade patients are more likely to suffer cognitive function decline, and the effect of hypnotic agents may vary.
CONCLUSION
This study concluded that the mean cognitive scores at one month following surgery were better maintained with propofol as compared to desflurane. In individual domain analysis, orientation and memory scores were better preserved with propofol compared to desflurane. Future large multicenter studies are needed to substantiate the findings of the present study.
Ethical approval
The study conformed to the standards of the Declaration of Helsinki and was approved by the Institute Ethics Committee (Reference no. NK/6671/DM/471).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adembri C, Venturi L, Pellegrini Giampietro DE. Neuroprotective effects of propofol in acute cerebral injury. CNS Drug Rev. 2007. 13: 333-5
2. Al-Khindi T, Loch Macdonald R, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010. 41: e519-36
3. Balasubramanian M, Kuberan A, Rawat A, Dhandapani S, Panda N, Kumar A. Effect of general anesthetics on caspase-3 levels in patients with aneurysmal subarachnoid hemorrhage: A preliminary study. J Neurosurg Anesthesiol. 2021. 33: 172-6
4. Bharati SJ, Mahajan C, Prabhakar H, Goyal K, Chowdhury T, Chandra H. Early cognitive functions in patients after embolization in neuroradiological suite-a comparison of two anesthetic techniques. J Anesth Clinic Res. 2011. 2: 3
5. Bhardwaj A, Bhagat H, Grover VK, Panda NB, Jangra K, Sahu S. Comparison of propofol and desflurane for postanaesthetic morbidity in patients undergoing surgery for aneurysmal SAH: A randomized clinical trial. J Anesth. 2018. 32: 250-8
6. Borsook D, George E, Kussman B, Becerra L. Anesthesia and perioperative stress: Consequences on neural network and postoperative behaviours. Prog Neurobiol. 2010. 92: 601-12
7. Canet J, Raeder J, Rasmussen LS, Enlund M, Kuipers HM, Hanning CD. Cognitive dysfunction after minor surgery in the elderly. Acta Anaesthesiol Scand. 2003. 47: 120410
8. Cheng H, Shi J, Zhou M. Cognitive assessment in Chinese patients with aneurysmal subarachnoid hemorrhage. Behav Neurol. 2006. 17: 117-20
9. De Santis A, Laiacona M, Barbarotto R, De Divitiis O, Migliore M, Capitani E. Neuropsychological outcome of operated cerebral aneurysms: Prognostic factors in 148 patients. Acta Neurol Scand. 1998. 97: 393-7
10. Feng HJ, Macdonald RL. Multiple actions of propofol on alpha beta gamma and alpha beta delta GABAA receptors. Mol Pharmacol. 2004. 66: 1517-24
11. Flutterer CD, Maurer MH, Schmitt A, Feldmann RE, Kuschinsky W, Washke KF. Alterations in the rat brain proteins after desflurane anesthesia. Anesthesiology. 2004. 100: 302-8
12. Hutter BO, Kreitschmann-Andermahr I, Mayfrank L, Rohde V, Spetzger U, Gilsbach JM. Functional outcome after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl (Wien). 1999. 72: 157-74
13. Joys S, Panda NB, Ahuja CK, Luthra A, Tripathi M, Mahajan S. Comparison of effects of propofol and sevoflurane on the cerebral vasculature assessed by digital subtraction angiographic parameters in patients treated for ruptured cerebral aneurysm: A preliminary study. J Neurosurg Anesthesiol. 2023. 1;35: 327-332.33
14. Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002. 33: 200-8
15. Lui X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG. Propofol disrupts functional interaction between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012. 33: 2487-98
16. Mahajan C, Chouhan RS, Rath GP, Dash HH, Suri A, Chandra SP. Effect of intraoperative brain protection with propofol on postoperative cognition in patients undergoing temporary clipping during intracranial aneurysm surgery. Neurol India. 2014. 62: 262-8
17. Mandal PK, Schifillliti D, Mafrica F, Fadale V. Inhaled anesthesia and cognitive performance. Drugs Today (Barc). 2009. 45: 47-54
18. Mielck F, Stephan H, Buhre W, Weyland A, Sonntag H. Effects of 1 MAC desflurane on cerebral metabolism, blood flow and carbon dioxide reactivity in humans. Br J Anaesth. 1998. 81: 155-60
19. Muzzi DA, Lossaso TJ, Dietz NM. The effect of desflurane & isoflurane on CSF pressure in humans with supratentorial mass lesions. Anaesthesiology. 1992. 76: 720-4
20. Nagashima K, Zorumski CF, Izumi Y. Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology. 2005. 103: 318-26
21. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collins I. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005. 53: 695-9
22. Ogden JA, Mee EW, Henning M. Prospective study of impairment of cognition and memory and recovery after subarachnoid hemorrhage. Neurosurgery. 1993. 33: 572-86
23. Patel PM, Drummond JC, Miller RD, editors. Cerebral physiology and the effects of anesthetic drugs. Miller’s anesthesia. Philadelphia, PA: Churchill Livingstone; 2009. 1: 305-40
24. Sharma N, Wig J, Mahajan S, Chauhan R, Mohanty M, Bhagat H. Comparison of postoperative cognitive dysfunction with the use of propofol versus desflurane in patients undergoing surgery for clipping of aneurysm after subarachnoid hemorrhage. Surg Neurol Int. 2020. 11: 174
25. Stenhouse LM, Knight RG, Longmore BE, Bishara SN. Long-term cognitive deficits in patients after surgery on aneurysms of the anterior communicating artery. J Neurol Neurosurg Psychiatry. 1991. 54: 909-14
26. Strebel S, Kaufman M, Guardiola PM, Schaefer HG. Cerebral vasomotor responsiveness is preserved during propofol and midazolam anaesthesia in humans. Anaesth Analg. 1994. 78: 884-8
27. Vandesteene A, Trempont V, Engelman E. Effect of propofol on cerebral blood flow and metabolism in man. Anaesthesia. 1988. 43: 42-3
28. Wei H, Xie Z. Anesthesia, calcium homeostatsis and Alzheimers disease. Curr Alzheimer Res. 2009. 6: 30-5
29. Weiss N, Sanchez P, Roche S, Beaudeux JL, Colonne C. S-100B as an additional prognostic marker in subarachnoid aneurysmal hemorrhage. Crit Care Med. 2006. 36: 2267-73
30. Wong GK, Lam S, Ngai K, Wong A, Mok V, Poon WS. Evaluation of cognitive impairment by the montreal cognitive assessment in patients with aneurysmal subarachnoid Hemorrhage: Prevalence, risk factors and correlations with 3-month outcomes. J Neurol Neurosurg Psychiatry. 2012. 83: 1112-7
31. Xie Z, Culley DJ, Dong Y, Zhang B, Moir RD. The common inhalational anesthetic isoflurane induces caspase activation and increases amyloid beta protein in vivo. Ann Neurol. 2008. 64: 618-27
32. Yin J, Wang SL, Lui XB. The effects of general anesthesia on memory in children: A comparison between propofol and sevoflurane. Anaesthesia. 2014. 69: 118-23