- Department of Neurosurgery, Saiseikai Shiga Hospital, Imperial Gift Foundation Inc., Shiga, Japan.
DOI:10.25259/SNI_640_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shigeomi Yokoya, Akihiko Hino, Yukihiro Goto, Hideki Oka. Complete relief of vasospasm – Effect of nicardipine coating during direct clipping for the patient with symptomatic vasospasm of subarachnoid hemorrhage. 18-Nov-2020;11:394
How to cite this URL: Shigeomi Yokoya, Akihiko Hino, Yukihiro Goto, Hideki Oka. Complete relief of vasospasm – Effect of nicardipine coating during direct clipping for the patient with symptomatic vasospasm of subarachnoid hemorrhage. 18-Nov-2020;11:394. Available from: https://surgicalneurologyint.com/surgicalint-articles/10397/
Abstract
Background: Some patients come to the hospital presenting with ischemic neurological deficits due to postsubarachnoid hemorrhage (SAH) cerebral vasospasm. In such a situation, neurosurgeons tend to avoid direct clipping, since mechanical irritation to the vessels could worsen the vasospasm and exacerbate ischemic symptoms. The optimal timing of direct clipping in patients with evidence of vasospasm is undetermined. Herein, we present the case of a patient who underwent direct clipping in the presence of severe symptomatic and post-SAH angiographic vasospasm. During surgery, we coated the severely spastic artery with nicardipine.
Case Description: A 49-year-old woman was admitted to our hospital with the diagnosis of ruptured intracranial aneurysm and severe vasospasm. On the admission day, we performed direct clipping together with direct application of nicardipine to the spastic artery. Postoperative immediate cerebral angiography showed complete disappearance of the vasospasm.
Conclusion: Direct clipping should not be contraindicated during the vasospasm period in patients with a ruptured aneurysm, and direct application of nicardipine on the spastic artery would completely relieve vasospasm.
Keywords: Cerebral vasospasm, Direct clipping, Nicardipine, Subarachnoid hemorrhage
INTRODUCTION
Most patients with aneurysmal subarachnoid hemorrhage (SAH) experience sudden, severe headache and come to the hospital on day 1. However, rarely, few patients come to the hospital due to ischemic neurological deficits during cerebral vasospasm post-SAH. In clinical practice, neurosurgeons tend to avoid direct clipping during cerebral vasospasm since mechanical irritation to the arteries could worsen vasospasm and exacerbate postoperative ischemic symptoms. Some studies reported that surgery during cerebral vasospasm increases the frequency of cerebral vasospasm and has a poor prognosis.[
Conversely, the timing of aneurysm surgery is reported to not influence the development of delayed cerebral ischemia;[
We herein report a successfully treated patient who underwent direct clipping with severe symptomatic and angiographic vasospasm after SAH. During surgery, we coated nicardipine to the artery where we suspected severe vasospasm. Postoperative cerebral angiography (CAG), performed immediately after the procedure, confirmed that the vasospasm was completely released.
CASE DESCRIPTION
A 49-year-old woman was referred to our hospital with complaints of general fatigue, sweating, and headache for 6 days and consciousness disturbance. She had right hand weakness for 1 day.
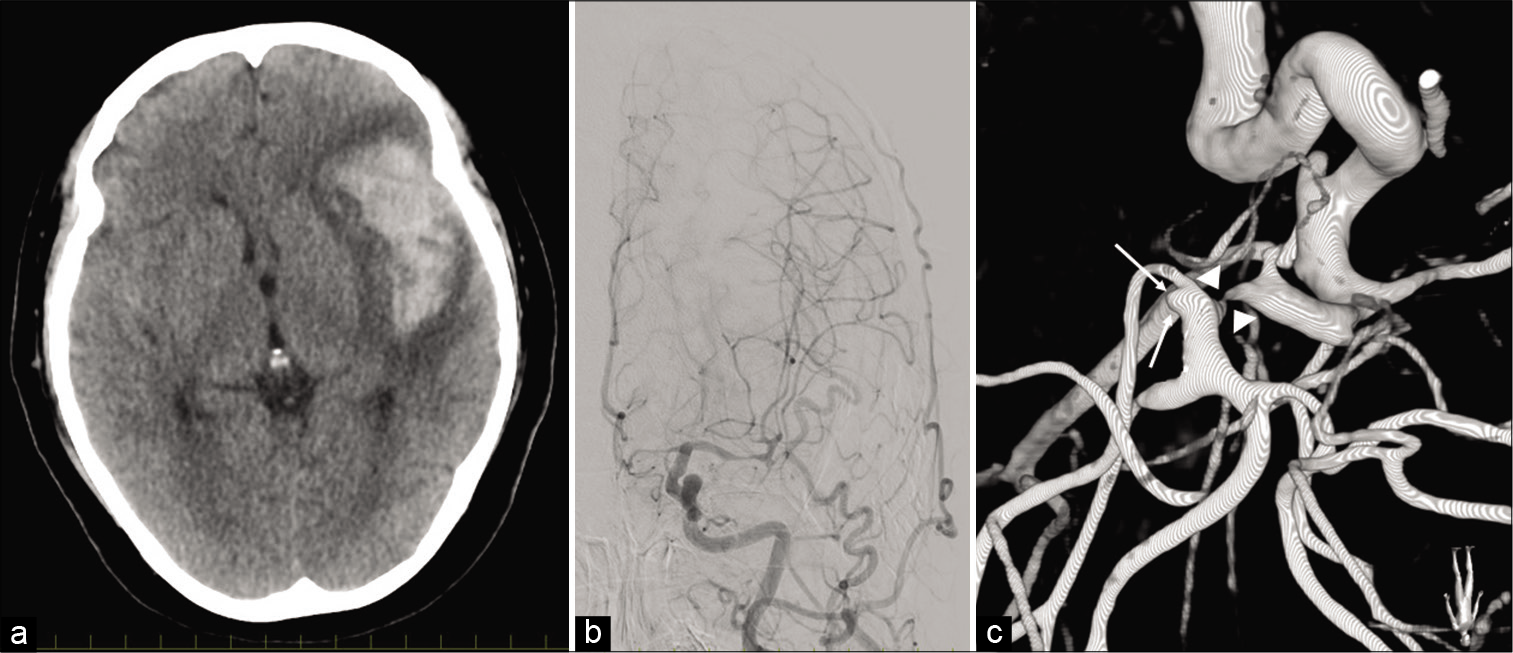
On admission, she had disturbance of consciousness (Glasgow Coma Scale, E4V4M6) with dysarthria, aphasia, and dexterity impairment of the right hand. Computed tomography (CT) demonstrated a localized clot in the left Sylvian fissure with perifocal edema [
Figure 1:
(a) Computed tomography, at admission, demonstrating that localized thick clot in the left Sylvian fissure. Note that it comes with perifocal edema. (b) Preoperative cerebral angiography demonstrating that the left middle cerebral artery and anterior cerebral artery showed evidence of severe vasospasm. (c) Three-dimensional digital subtraction angiography, operative view, demonstrating that small aneurysm originates from the left sphenoidal segment of middle cerebral artery (M1) – insular segment of middle cerebral artery (M2) bifurcation (arrow). Note that severe vasospasm was observed in M1 (arrow head).
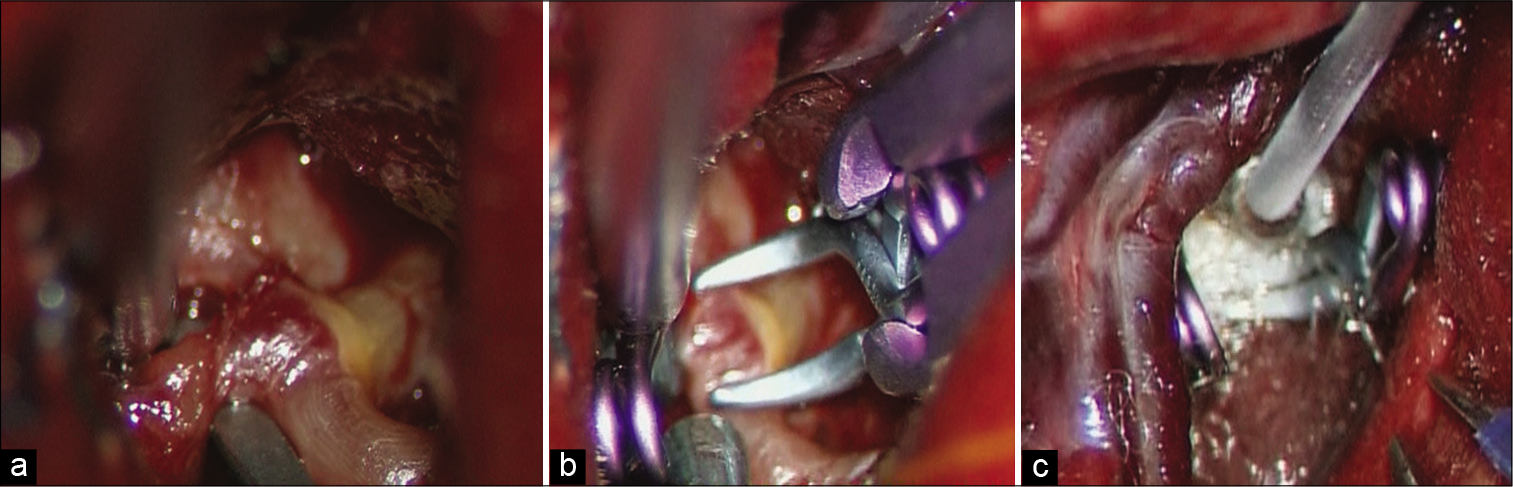
During surgery, after the ruptured aneurysm was clipped [
Figure 2:
(a) Intraoperative photograph during direct clipping demonstrating that the left M1-M2 bifurcation aneurysm was exposed. A fibrin cap covers the dome of the aneurysm and the source of intra-Sylvian hematoma was determined to be this small aneurysm. (b) The ruptured aneurysm was occluded with two clips. (c) After clipping of the aneurysm, spastic vessels were directly applied with nicardipine which was soaked in oxidized cellulose.
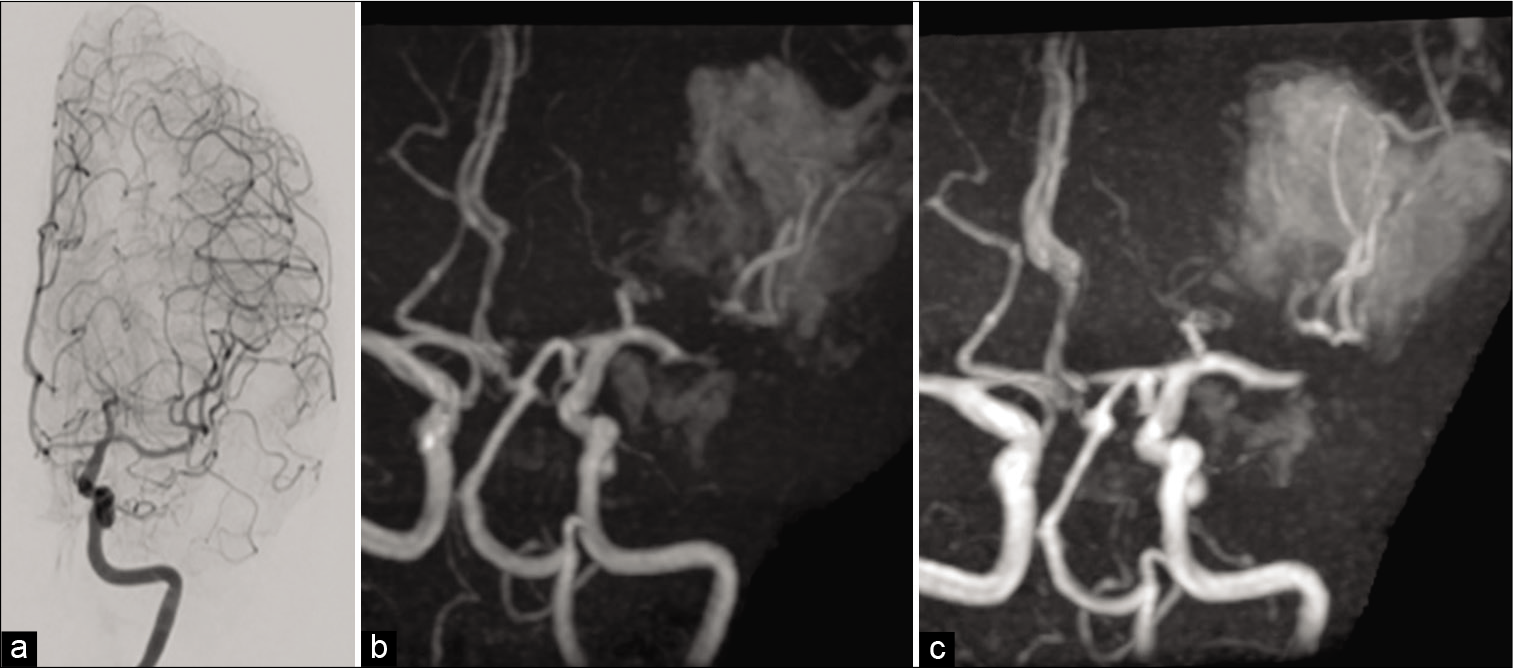
Figure 3:
(a) Postoperative cerebral angiography, performed just after the direct clipping of the left middle cerebral artery (MCA) aneurysm, demonstrating that the left M1 was completed dilated. (b) Magnetic resonance angiography (MRA), on the 3rd day of operation, demonstrating that the left MCA showed evidence of vasospasm. (c) MRA, on the 18th day of the operation, demonstrating no evidence of vasospasm on the left anterior cerebral artery and MCA.
Postoperatively, the patient received an intravenous infusion of ozagrel sodium at 80 mg/day, an intravenous infusion of nicardipine at 24 mg/day, an intravenous drip of fasudil hydrochloride at 90 mg/day, and a maintenance intravenous Vitamin B1-containing infusion (BFLUID® Otsuka Pharmaceutical Co., Ltd., Tokushima, Japan) 1000 mL/day.
Although her symptoms did not deteriorate after surgery, magnetic resonance angiography (MRA) performed 3 days after the operation showed reappearance of vasospasm [
DISCUSSION
This case revealed that vasospasm could be markedly relieved by placing oxidized cellulose soaked with nicardipine solution to the arteries during the procedure at least transitionally. Nicardipine is reported to have an effect on the prevention of symptomatic vasospasm after SAH. A few RCTs demonstrated that intravenous nicardipine (0.15 mg/kg/h) could significantly reduce vasospasm incidence,[
However, a precaution should be taken in the direct application of nicardipine. The effect may be transitory, and vasospasm may reappear. As shown in
You may argue that endovascular approaches may be a mainstream option, as some reports demonstrated that the timing of endovascular treatment for ruptured intracranial aneurysms does not raise the occurrence of clinical vasospasm and/or outcomes.[
However, endovascular treatment is not suitable in all cases. The wide neck, small aneurysms at the distal arterial tree, as in this case, does not appear to facilitate complete occlusion. In addition, there are complications associated with the endovascular procedure. The clinical study for analyzing the occurrence and outcome of complications in the endovascular treatment showed that the endovascular approach has an approximately 4.4% risk of intraoperative rupture (notably, ruptured middle cerebral artery aneurysms have an intraoperative rupture risk as high as 8.5%) and 12.5% risk of thromboembolic events.[
Compared to endovascular surgery, direct clippings have several advantages. The risk of rebleeding after the direct procedure is lower than that in endovascular procedure. If the aneurysm ruptures intraoperatively, we can take more measures to deal with it during direct clipping than when we can take during endovascular treatment. The disadvantage we fear most about direct clipping during vasospasm is that surgical manipulation of the spastic arteries may aggravate arterial narrowing, which results in postoperative ischemic deficits. However, direct application of nicardipine relieved the vasospasm and may no longer be contraindicated for the patient, even in the presence of symptomatic and/or angiographical severe vasospasm.
The limitation of this study is that we experienced only one case where nicardipine was directly applied to the spastic artery; therefore, we could not draw a preferable general conclusion. However, our experience encourages rescue measures in direct clipping, with the assumption that it would be a preferable treatment option for similar cases.
CONCLUSION
Direct application of nicardipine for the spastic artery completely relieved the vasospasm, thus, supporting the view that direct clipping is not a contraindication for the ruptured aneurysm in patients with vasospasm.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Ethical standards
The publication of this case report was approved by the Ethics Committee of Saiseikai Shiga Hospital (Permission number: 433).
References
1. Badjatia N, Topcuoglu MA, Pryor JC, Rabinov JD, Ogilvy CS, Carter BS. Preliminary experience with intra-arterial nicardipine as a treatment for cerebral vasospasm. AJNR Am J Neuroradiol. 2004. 25: 819-26
2. Baltsavias GS, Byrne JV, Halsey J, Coley SC, Sohn MJ, Molyneux AJ. Effects of timing of coil embolization after aneurysmal subarachnoid hemorrhage on procedural morbidity and outcomes. Neurosurgery. 2000. 47: 1320-9
3. Barth M, Capelle HH, Weidauer S, Weiss C, Münch E, Thomé C. Effect of nicardipine prolonged-release implants on cerebral vasospasm and clinical outcome after severe aneurysmal subarachnoid hemorrhage: A prospective, randomized, double-blind phase IIa study. Stroke. 2007. 38: 330-6
4. de Gans K, Nieuwkamp DJ, Rinkel GJ, Algra A. Timing of aneurysm surgery in subarachnoid hemorrhage: A systematic review of the literature. Neurosurgery. 2002. 50: 336-40
5. Elliott JP, Newell DW, Lam DJ, Eskridge JM, Douville CM, Le Roux PD. Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 1998. 88: 277-84
6. Gruber A, Ungersböck K, Reinprecht A, Czech T, Gross C, Bednar M. Evaluation of cerebral vasospasm after early surgical and endovascular treatment of ruptured intracranial aneurysms. Neurosurgery. 1998. 42: 258-67
7. Haley EC, Kassell NF, Torner JC. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the cooperative aneurysm study. J Neurosurg. 1993. 78: 537-47
8. Haley EC, Kassell NF, Torner JC. The international cooperative study on the timing of aneurysm surgery. The North American experience. Stroke. 1992. 23: 205-14
9. Hayashi K, Hirao T, Sakai N, Nagata I. Current status of endovascular treatment for vasospasm following subarachnoid hemorrhage: Analysis of JR-NET2. Neurol Med Chir (Tokyo). 2014. 54: 107-12
10. Kassell NF, Torner JC, Haley EC, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: Overall management results. J Neurosurg. 1990. 73: 18-36
11. Kassell NF, Torner JC, Jane JA, Haley EC, Adams HP. The international cooperative study on the timing of aneurysm surgery. Part 2: Surgical results. J Neurosurg. 1990. 73: 37-47
12. Kasuya H, Onda H, Takeshita M, Okada Y, Hori T. Efficacy and safety of nicardipine prolonged-release implants for preventing vasospasm in humans. Stroke. 2002. 33: 1011-5
13. Murayama Y, Malisch T, Guglielmi G, Mawad ME, Viñuela F, Duckwiler GR. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms: Report on 69 cases. J Neurosurg. 1997. 87: 830-5
14. Pierot L, Cognard C, Anxionnat R, Ricolfi F. Ruptured intracranial aneurysms: Factors affecting the rate and outcome of endovascular treatment complications in a series of 782 patients (clarity study). Radiology. 2010. 256: 916-23
15. Polin RS, Coenen VA, Hansen CA, Shin P, Baskaya MK, Nanda A. Efficacy of transluminal angioplasty for the management of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000. 92: 284-90
16. Solomon RA, Onesti ST, Klebanoff L. Relationship between the timing of aneurysm surgery and the development of delayed cerebral ischemia. J Neurosurg. 1991. 75: 56-61
17. Velat GJ, Kimball MM, Mocco JD, Hoh BL. Vasospasm after aneurysmal subarachnoid hemorrhage: Review of randomized controlled trials and meta-analyses in the literature. World Neurosurg. 2011. 76: 446-54
18. Yalamanchili K, Rosenwasser RH, Thomas JE, Liebman K, McMorrow C, Gannon P. Frequency of cerebral vasospasm in patients treated with endovascular occlusion of intracranial aneurysms. AJNR Am J Neuroradiol. 1998. 19: 553-8
19. Zwienenberg-Lee M, Hartman J, Rudisill N, Madden LK, Smith K, Eskridge J. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher Grade III subarachnoid hemorrhage: Results of a Phase II multicenter, randomized, clinical trial. Stroke. 2008. 39: 1759-65