- Department of Neurosurgery, Yashoda Hospitals, Hyderabad, Telangana, India.
Correspondence Address:
Kartik Chandra, Resident, Department of Neurosurgery, Yashoda Hospitals, Hyderabad, Telangana, India.
DOI:10.25259/SNI_861_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kartik Chandra, B. J. Rajesh. Concomitant extradural, subdural, and intraparenchymal abscesses of the brain in a patient with cerebral melioidosis – A case report. 23-Dec-2022;13:588
How to cite this URL: Kartik Chandra, B. J. Rajesh. Concomitant extradural, subdural, and intraparenchymal abscesses of the brain in a patient with cerebral melioidosis – A case report. 23-Dec-2022;13:588. Available from: https://surgicalneurologyint.com/surgicalint-articles/12075/
Abstract
Background: Extra axial abscess of the brain is a rare entity, moreover, extra-axial abscess concomitant with intraparenchymal purulent collections are scarcely reported in the literature. Etiology includes penetrating trauma, paranasal sinusitis, mastoiditis, craniospinal surgeries, and the rare spread of infectious agents through the hematogenous route.
Case description: We present a case of a young male with Burkholderia pseudomallei Central Nervous System (CNS) melioidosis, forming abscesses in extra-axial and intraparenchymal planes without contiguity.
Conclusion: This is to emphasize the importance of MR spectroscopy and other convenient methods in differentiating the etiology in cranial infections.
Keywords: Abscess, Burkholderia, Epidural, Intraparenchymal, Melioidosis, Subdural
INTRODUCTION
Purulent collections in the central nervous system (CNS) occur in the spaces between the inner table of the skull and dura; between the dura and arachnoid mater, known as epidural, and subdural spaces, respectively, collectively referred to as extra-axial abscesses. Furthermore, abscess formation can also occur intraparenchymal known as an intra-axial abscess. Developing countries have a higher incidence of abscesses, around 8%, of all CNS space-occupying lesions. Of the micro-organisms affecting the brain, Gram-negative bacilli account for 9–31% of the total infections.[
CASE REPORT
A 24-year-old male patient presented to us with global aphasia and right-sided hemiplegia. Medical history was significant for intermittent headaches for 4 months. One month after the onset of symptoms, the patient developed an evening rise in temperatures and abdominal pain. He sought treatment at another institution where he was evaluated with ultrasound abdomen that showed splenomegaly with multiple granulomas and the findings were later confirmed with computerized tomography (CT) abdomen which also revealed periportal, para-aortic, and aortic caval lymphadenitis-like features, for which he was treated presumptively as a mycobacterial tuberculosis infection. Ten days later, the symptoms worsened and the patient developed new-onset speech difficulty along with right-sided weakness. Consequently, the patient was evaluated with a magnetic resonance imaging (MRI) brain which was suggestive of left frontal thin subdural (measuring around 2*3*2 mm, volume 6 cubic mm) and a rim of thin epidural collection, and the patient was continued on anti-tubercular treatment (ATT) presuming CNS tuberculosis.
A month later, he presented to us when complaints progressed to aphasia and hemiplegia. Physical examination was significant for altered sensorium with a Glasgow Coma Scale of E3VAM5 and right-sided hemiplegia. The patient was reevaluated with a plain and contrast MRI of the brain. On T1, isointense rim with the hypointense collection was noted in the lesion [
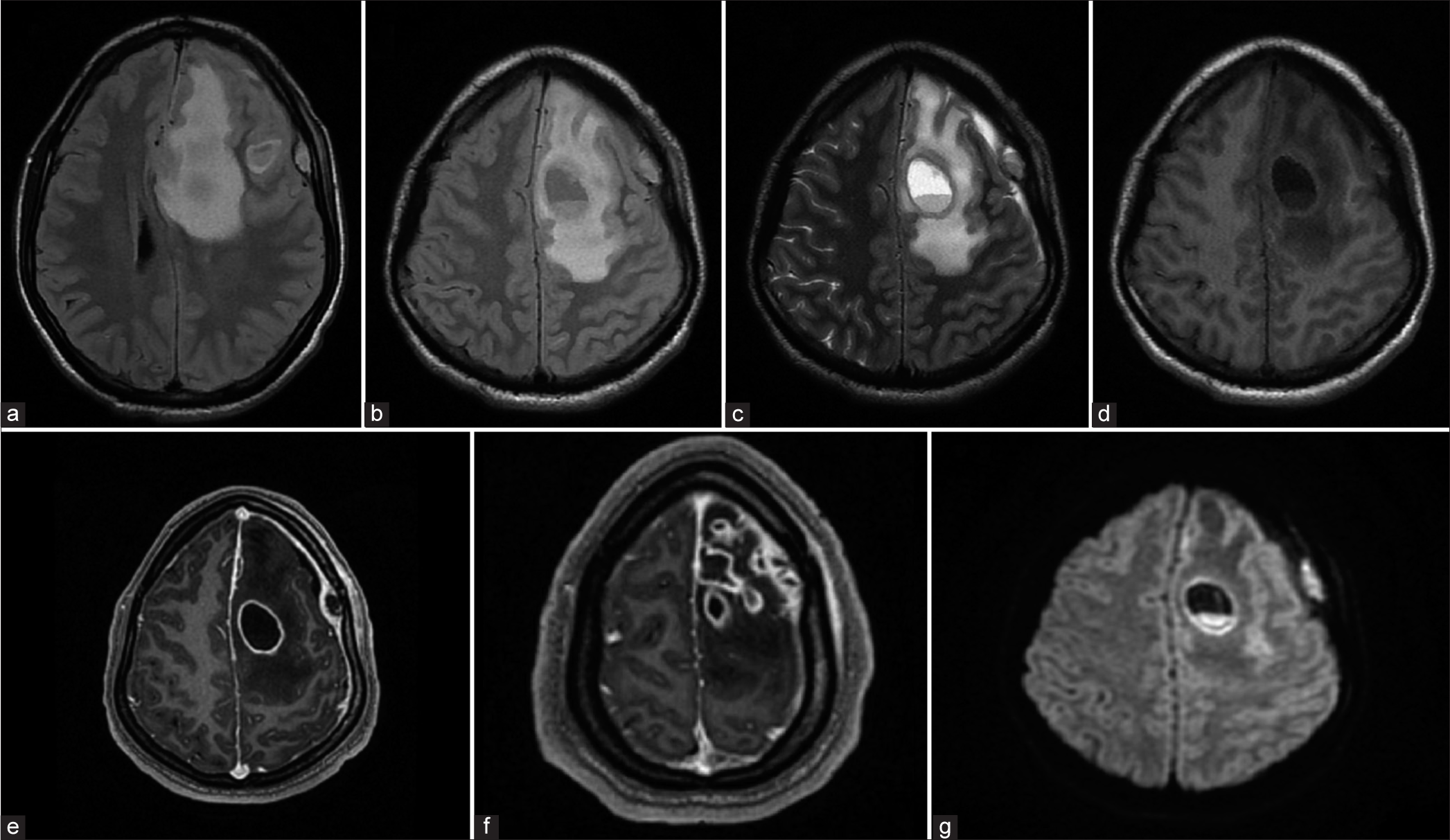
Figure 1:
MRI findings (a) MRI T1 showing intraparenchymal abscess and edema causing mass effect, (b) MRI T1 fluid-fluid level in the abscess, (c) MRI T2 shows hyperintense collection in subdural and epidural space, (d) MR Flair shows hyperintense extra-axial collection, (e and f) MR Contrast showing multiple ring-enhancing lesions, sub dural, and epi dural collections, (g) Diffusion restriction noted along the capsule and contents of the abscess.
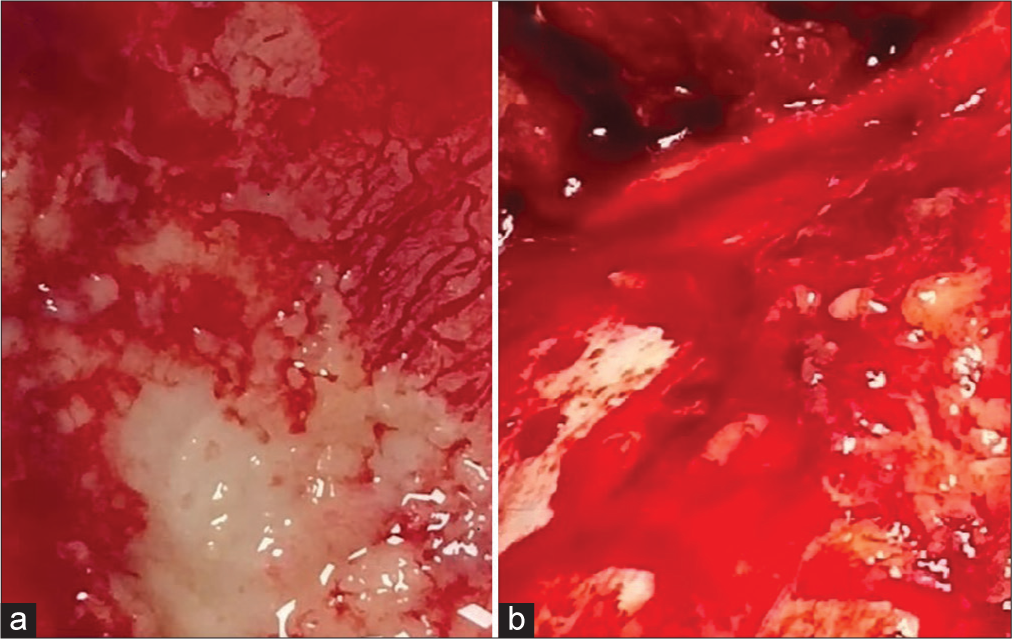
The patient underwent left fronto-temporo-parietal decompressive craniectomy and abscess evacuation. After craniectomy, adherent purulent collection was noted on the inner table of the bone flap and dura, suggestive of osteomyelitis and epidural abscess [
DISCUSSION
CNS infections are caused by wide variety of organisms including bacteria, parasites, fungi, and viruses. The most common route for cerebral fungal abscess is systemic foci spreading to CNS through hematogenous route, commonly isolated organisms being Candida and Aspergillus species. Radiological features are multifocal lesions, involvement of deep grey matter nuclei, signal heterogeneity on diffusion weighted (DW) imaging and higher apparent diffusion coefficient (ADC) values. Other features include meningitis, multiple infarcts, thrombosis, and aneurysm formation all attributable to vascular invasion by fungal elements.[
Melioidosis is caused by a Gram-negative aerobic motile bacillus, B. pseudomallei, endemic to regions like Australia and parts of South-east Asia like India.[
The purulent collection of the subdural space and the intraparenchymal abscesses resulted in significant vasogenic edema in the left frontal lobe causing aphasia and right-sided weakness in our patient. B. pseudomallei crosses the blood-brain barrier using the transcellular, paracellular, or trojan horse methods.[
The clinical signs of raised intracranial pressure such as altered sensorium, alongside radiological findings of vasogenic edema leading to mass effect and involvement of bone by epidural abscess warranted management with decompressive craniectomy over conventional craniotomy and abscess evacuation. Intraoperatively, following the retrieval of the bone flap, pyogenic material was noted over the dura and the inner table of the bone suggesting epidural empyema. The valveless nature of the diploic veins facilitated the retrograde spread of infection to the epidural space.[
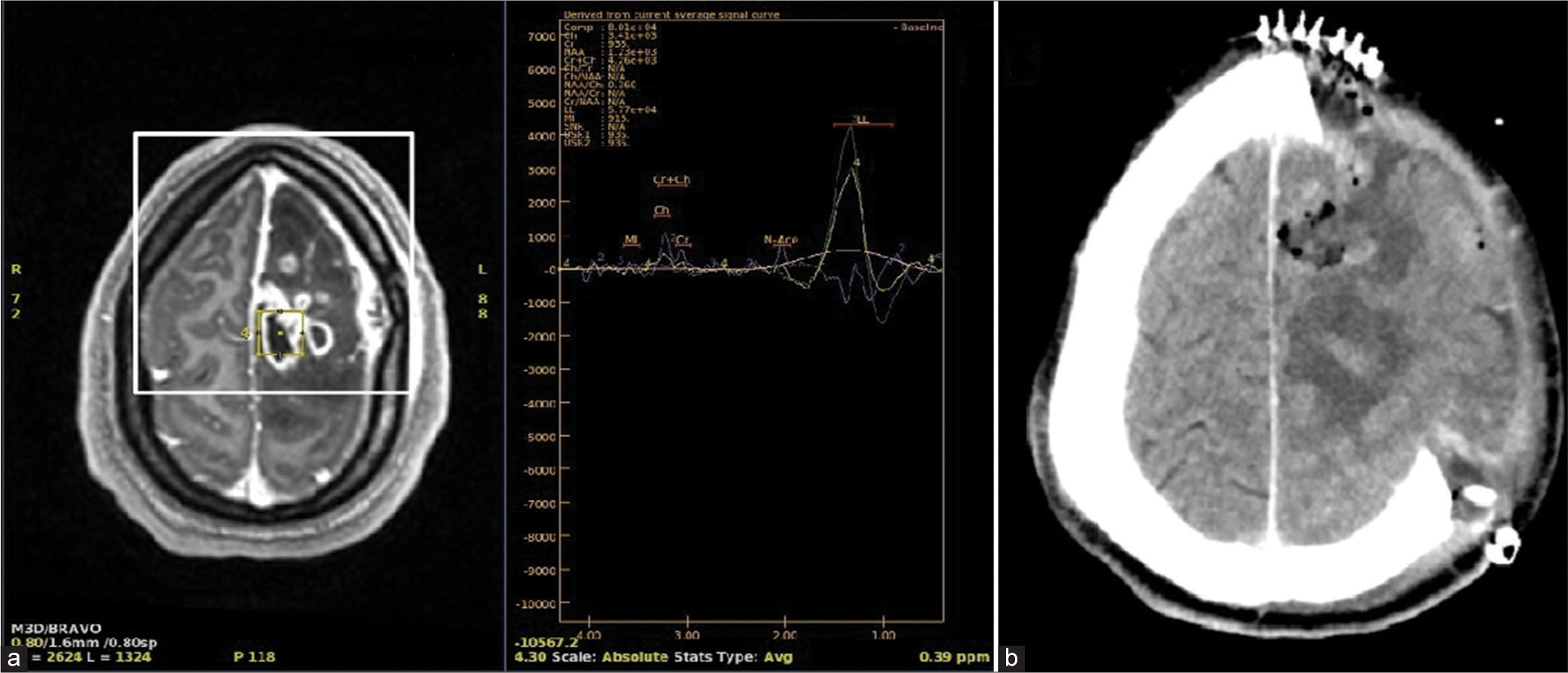
Role of MR spectroscopy in infectious etiology: Cerebral Bacterial abscess usually show peaks of amino-acids, acetate, aspartate, and succinate peaks with marked decline of neuronal markers such as N-acetyl aspartate, creatine, and choline. Closest differential diagnosis to bacterial abscess is cystic glioma and cystic metastasis, radiologically they can be distinguished by ADC values and DWI sequence, where bacterial abscess features higher diffusion restriction on DWI and lower ADC values, whereas neoplastic etiology usually features moderate diffusion restriction and higher ADC values.[
Our case report also identifies the need for convenient methods to differentiate between TB and other bacterial abscesses. The radiological impression at the time of presentation seen in the patient was closely mimicking tuberculosis abscess prompting the initiation of ATT without a definitive diagnosis. At present, the most effective method to differentiate between tuberculous and bacterial abscesses is MR spectroscopy.[
CONCLUSION
This is to emphasize the importance of MR spectroscopy and other convenient methods in differentiating the etiology in cranial infections.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Andreula C. Cranial viral infections in the adult. Eur Radiol. 2004. 14: E132-44
2. Currie BJ, Fisher DA, Howard DM, Burrow JN. Neurological melioidosis. Acta Trop. 2000. 74: 145-51
3. El-Feky MElBeialy M. Brain Abscess-MR Spectroscopy. Available from: https://www.radiopaedia.org [Last accessed on 2022 Sep 16].
4. Fountas KN, Duwayri Y, Kapsalaki E, Dimopoulos VG, Johnston KW, Peppard SB. Epidural intracranial abscess as a complication of frontal sinusitis: Case report and review of the literature. South Med J. 2004. 97: 279-82 quiz 283
5. Gupta RK, Vatsal DK, Husain N, Chawla S, Prasad KN, Roy R. Differentiation of tuberculous from pyogenic brain abscesses with in vivo proton MR spectroscopy and magnetization transfer MR imaging. AJNR Am J Neuroradiol. 2001. 22: 1503-9
6. Kumar GS, Raj PM, Chacko G, Lalitha MK, Chacko AG, Rajshekhar V. Cranial melioidosis presenting as a mass lesion or osteomyelitis. J Neurosurg. 2008. 108: 243-7
7. Kural C, Kırmızıgoz S, Ezgu MC, Bedir O, Kutlay M, Izci Y. Intracranial infections: Lessons learned from 52 surgically treated cases. Neurosurg Focus. 2019. 47: E10
8. Limmathurotsakul D, Peacock SJ. Melioidosis: A clinical overview. Br Med Bull. 2011. 99: 125-39
9. Mueller-Mang C, Castillo M, Mang TG, Cartes-Zumelzu F, Weber M, Thurnher MM. Fungal versus bacterial brain abscesses: Is diffusion-weighted MR imaging a useful tool in the differential diagnosis?. Neuroradiology. 2007. 49: 651-7
10. Ravikumar R, John DV. Brain abscess in the current decade (2010-2019) in India-a review. Ind J Neurosurg. 2021. 10: 95-102
11. Weingarten K, Zimmerman RD, Becker RD, Heier LA, Haimes AB, Deck MD. Subdural and epidural empyemas: MR imaging. AJR Am J Roentgenol. 1989. 152: 615-21
12. Wongwandee M, Linasmita P. Central nervous system melioidosis: A systematic review of individual participant data of case reports and case series. PLoS Negl Trop Dis. 2019. 13: e0007320