- Department of Neurosurgery, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
- College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- King Abdullah International Medical Research Center, Riyadh, Saudi Arabia.
Correspondence Address:
Wafa Faisal Aldhafeeri, Department of Neurosurgery, Prince Sultan Military Medical City, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_642_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Wafa Faisal Aldhafeeri1, Rahaf Farhan Alanazi2,3, Jamal Abdullah1, Abdullrahman Nazer1. Concurrent glossopharyngeal neuralgia and oromandibular dystonia resolved after microvascular decompression of the trigeminal and glossopharyngeal nerve: A rare presentation. 19-Apr-2024;15:132
How to cite this URL: Wafa Faisal Aldhafeeri1, Rahaf Farhan Alanazi2,3, Jamal Abdullah1, Abdullrahman Nazer1. Concurrent glossopharyngeal neuralgia and oromandibular dystonia resolved after microvascular decompression of the trigeminal and glossopharyngeal nerve: A rare presentation. 19-Apr-2024;15:132. Available from: https://surgicalneurologyint.com/surgicalint-articles/12867/
Abstract
Background: This type of pain syndrome occurs suddenly and briefly, beginning unilaterally from one side of the face. Modestly stimulating speech can provoke it, affecting the ear, tongue, throat, and jaw angle. Interestingly, it is the sensory distribution of the auricular and the pharyngeal branches of the cranial nerves IX and X. People have not had a confirmed case of glossopharyngeal neuralgia (GPN), along with oromandibular dystonia (OMD). Nevertheless, usually in the medical literature, this case report supplies information about a patient who has concurrent GPN and OMD.
Case Description: A 36-year-old male patient presented with a history of sudden onset of increasing electric pains, which were centered in the middle of the forehead to the depth of the throat and accompanied by uncontrolled movements, repetitive tongue protrusions, jaw movements, and recurrent pervasive gagging reflexes. Magnetic resonance imaging showed that a vascular loop of the superior cerebellar and anterior inferior cerebellar artery on the left side had crossed over and compressed those nerves. Decompression surgery in the left glossopharyngeal and trigeminal nerves cured all the symptoms.
Conclusion: The simultaneous occurrence of GPN and OMD is rare, complex, and challenging from the clinician’s viewpoint in the management of similar but different pathologies. A detailed history was taken, and a radiological investigation was called to devise a management plan in the context of understanding the pathology of both disorders.
Keywords: Glossopharyngeal neuralgia (GN), Microvascular decompression (MVD), Oromandibular dystonia (OD)
INTRODUCTION
Glossopharyngeal neuralgia (GPN) is an episodic syndrome estimated to account for an incidence of 0.2–1.3% of all cases of cranial neuralgia.[
CASE PRESENTATION
History for a 36-year-old previously medically free man was presented in the clinic with a long-standing history of abnormal facial movements and facial pain. He was labeled rediagnosed by many neurologists as trigeminal neuralgia (TGN) and underwent a trial line of medical therapy without a pronounced response. A case is presented with sudden electric pain in the middle of the forehead accompanied by a feeling of this kind down in the throat followed by an involuntary uncontrolled movement as repetitive gag reflexes with tongue protrusion and jaw movements lasting only a second. These symptoms are usually precipitated by eating and drinking and worsen at nighttime. The clinical picture of the case showed a string susceptibility of cooccurrence of OMD and GPN. Accordingly, the treatment plan included adding the Movement Disorder Neurologist, and the patient had been initiated on clonazepam. During his follow-up at the clinic, his involuntary movements came under control; however, due to the tapering down of other medicines, the electrical pain component overrode it.
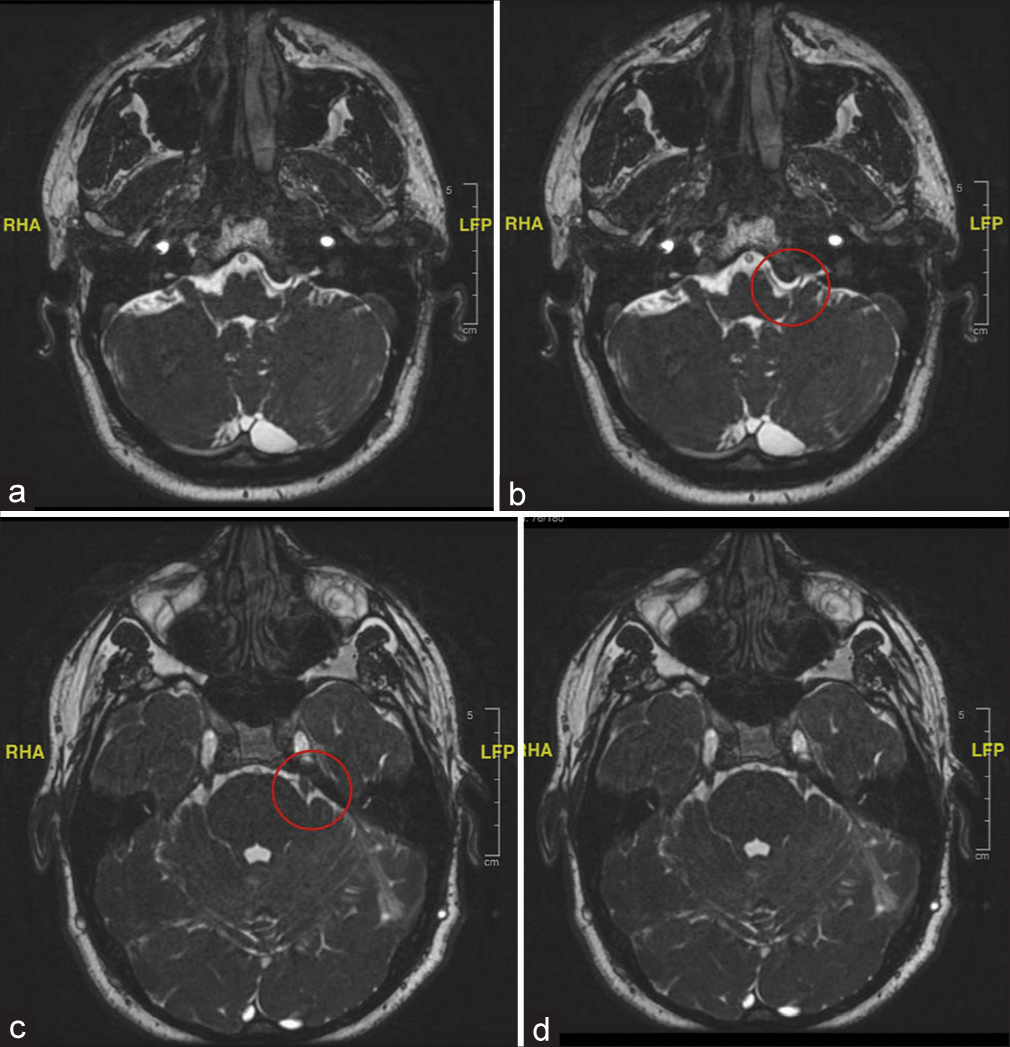
The radiological images in the multiple magnetic resonance imaging, magnetic resonance angiography, and fast imaging employing steady-state acquisition images indicated that there existed a constant offending vascular loop of the superior cerebellar artery as well as that of the anterior inferior cerebellar artery, crossing over to cause compression of the glossopharyngeal and the trigeminal nerve on the left side of the individual [
Figure 1:
(a and b) Magnetic resonance imaging FIESTA sequence: at the level of glossopharyngeal nerve on the left side consistent with an offending loop by both SCA and AICA causing compression at root entry zone and crossing over of glossopharyngeal nerve. Here, the red circle depicts the above-mentioned findings. (c and d) Magnetic resonance imaging FIESTA sequence: at the level of Meckel cave consistent with an offending loop by both SCA and AICA causing compression at root entry zone and crossing over of trigeminal nerve. Here, the red circle depicts the above-mentioned findings. FIESTA: Fast imaging employing steady-state acquisition, SCA: Superior cerebellar artery, AICA: Anterior inferior cerebellar artery.
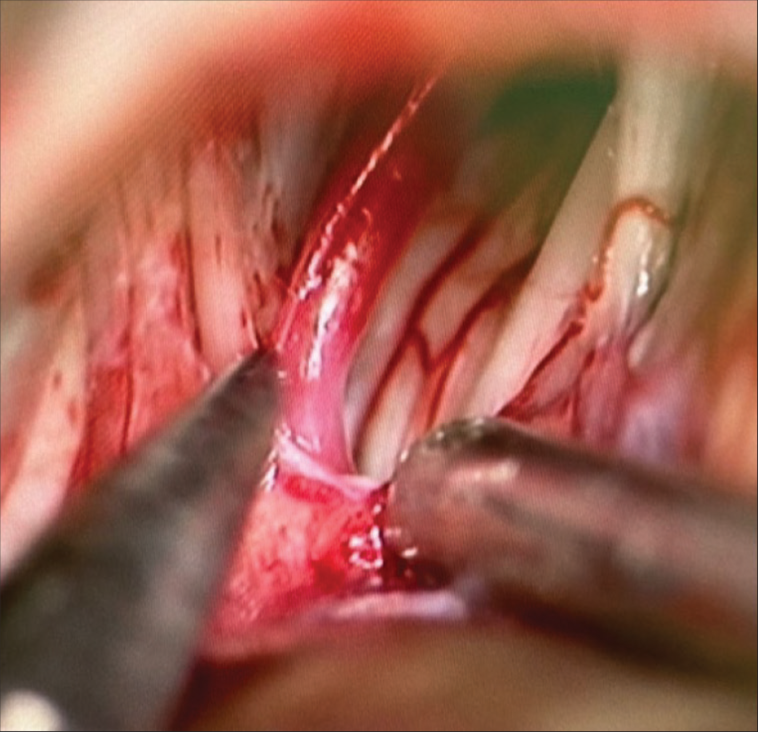
Microvascular decompression over the left side of the glossopharyngeal nerve, together with the trigeminal nerve, was done. Intraoperative findings were offending vessels, which were isolated and dissected away from the nerves by Teflon material application [
The patient gradually improved postoperatively till the complete resolution of his symptoms after one year. The patient was following up regularly, with a trial of weaning his current medicines for about a year without any relapse.
DISCUSSION
Trigeminal and glossopharngeal neurolgia accounts for only 5% of all GPN patients.[
Of course, it might be acceptable to approach microvascular decompression for GPN as a second-line treatment when standard medical treatment, for some reason, did not give the expected results if the pain relief was not achieved or the side effects of the drugs became unbearable. Appropriate specialized magnetic resonance imaging investigations are indispensable to visualize neurovascular compression in the root entry zone of the cranial nerve. Experienced neurologists request these and include 3D-constructive interference in the steady state.
Gaul et al. reported pain resolution in 16 of the 18 patients with GPN following MVD.[
At present, the pathophysiology of OMD remains to be unraveled. Trauma peripherally is considered one of the predisposing factors for several neurological disorders, including OMD.[
CONCLUSION
In conclusion, the simultaneous occurrence of Glossopharyngeal Neuralgia (GPN) and Oromandibular Dystonia (OMD) presents a rare and complex challenge for clinicians. This case study highlights the importance of a thorough history and radiological investigation in understanding and managing these disorders. Microvascular decompression surgery effectively relieved the symptoms in this patient, underscoring the significance of a multidisciplinary approach in treatment planning. While GPN typically responds well to microvascular decompression, the pathophysiology of OMD remains less understood, necessitating further research. Overall, this case emphasizes the need for tailored treatment strategies guided by the individual characteristics and underlying pathology of each disorder.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bruyn GW. Glossopharyngeal neuralgia. Cephalalgia. 1983. 3: 143-57
2. Gaul C, Hastreiter P, Duncker A, Naraghi R. Diagnosis and neurosurgical treatment of glossopharyngeal neuralgia: Clinical findings and 3-D visualization of neurovascular compression in 19 consecutive patients. J Headache Pain. 2011. 12: 527-34
3. Jankovic J, Van Der Linden C. Dystonia and tremor induced by peripheral trauma: predisposing factors. J Neurol Neurosurg Psychiatry. 1988. 51: 1512-9
4. Katusic S, Willaims DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945-1984. Neuroepidemiology. 1991. 10: 276-81
5. Patel A, Kassam A, Horowitz M, Chang YF, Barrow DL, Sindou M. Microvascular decompression in the management of glossopharyngeal neuralgia: Analysis of 217 cases. Neurosurgery. 2002. 50: 705-11
6. Raoofi S, Khorshidi H, Najafi M. Etiology, diagnosis and management of oromandibular dystonia: An update for stomatologists. J Dent. 2017. 18: 73-81
7. Rozen TD. Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin. 2004. 22: 185-206
8. Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia: A study of 217 cases. Arch Neurol. 1981. 38: 201-5
9. Sampson JH, Grossi PM, Asaoka K, Fukushima T, Casey KF, Jannetta PJ. Microvascular decompression for glossopharyngeal neuralgia: Long-term effectiveness and complication avoidance. Neurosurgery. 2004. 54: 884-90
10. Sankhla C, Lai EC, Jankovic J. Peripherally induced oromandibular dystonia. J Neurol Neurosurg Psychiatry. 1998. 65: 722-8
11. Skármeta NP, Espinoza-Mellado P, PedroChana Skármeta NP, Espinoza-Mellado P, Chana P, editors. Orofacial dystonia and other oromandibular movement disorders. Dystonia: Different prospects. London: Intechopen; 2018. p.