- Department of Neurosurgery (Trauma and Emergency), All India Institute of Medical Sciences, Raipur, Chhattisgarh, India.
- Department of Neurosurgery, Fortis Memorial Research Institute, Gurgaon, Haryana, India.
Correspondence Address:
Sanjeev Sreenivasan, Department of Neurosurgery (Trauma and Emergency), All India Institute of Medical Sciences, Raipur, Chhattisgarh, India.
DOI:10.25259/SNI_132_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sanjeev Sreenivasan1, Chinmay Arora2, Sandeep Vaishya2, Rana Patir2. Concurrent intracranial infarct and intraventricular hemorrhage with spontaneous nontraumatic extradural hemorrhage in follow-up: An enigma of COVID-19-associated intracranial vasculopathy. 11-Mar-2022;13:90
How to cite this URL: Sanjeev Sreenivasan1, Chinmay Arora2, Sandeep Vaishya2, Rana Patir2. Concurrent intracranial infarct and intraventricular hemorrhage with spontaneous nontraumatic extradural hemorrhage in follow-up: An enigma of COVID-19-associated intracranial vasculopathy. 11-Mar-2022;13:90. Available from: https://surgicalneurologyint.com/surgicalint-articles/11432/
Abstract
Background: Several neurological manifestations have been described in the literature, in patients affected with COVID-19 infection. Some common forms include ischemic stroke, cardioembolic stroke, intraparenchymal hemorrhage, and multicompartmental hemorrhage. Concurrent brain infarct and intraventricular hemorrhage (IVH) have not been described in the literature previously.
Case Description: A 35-year hypertensive and COVID-19-positive patient developed sudden-onset spontaneous IVH with concurrent infarct in the left internal capsule. In spite of undergoing an initial CSF drainage procedure, he had persistent worsening sensorium and increasing midline shift on CT imaging, so he underwent a left-sided decompressive craniectomy. One month after discharge, he developed spontaneous extradural hemorrhage at the operative site. In view of impending cerebral herniation, emergency hematoma evacuation was done, which restored his neurological status.
Conclusion: This is the first reported detailed case of concurrent intracranial infarct and IVH in a patient affected with COVID-19 infection. We also report a rare phenomenon of nontraumatic noncoagulopathic extradural hemorrhage on the decompressive craniectomy site, in this patient 1 month after surgery.
Keywords: Brain infarct, COVID-19, Intraventricular hemorrhage, Spontaneous extradural hemorrhage
INTRODUCTION
Although multicompartmental intracranial hemorrhage has been reported in the past, simultaneous occurrence of intracranial infarct and intraventricular hemorrhage (IVH) has not been detailed in the literature. Spontaneous extradural hemorrhage has been reported in patients on treatment with anticoagulants/antiplatelets. Occurrence of this phenomenon in a hypertensive and COVID-19-positive patient, in the absence of such medication, presents an interesting challenge for surgical management.
CASE DETAILS
A 35-year male patient presented with sudden-onset altered sensorium and right-sided hemiparesis for 6 h. There was no history of hypertension (HTN), diabetes mellitus (DM), coronary artery disease, and recurrent transient ischemic attacks.
He had been treated for pneumonia secondary to coronavirus-2019 (SARS-CoV-2) infection (COVID-19) 4 weeks ago, when he was admitted with cough and breathlessness. A positive reverse transcriptase polymerase chain reaction test for COVID-19 infection was noticed. Serum assays reported D-dimer level of 0.6 ug/ml (normal) and fibrinogen level of 4.2 g/L (normal range <7 g/L). Total leukocyte count, platelet count, troponin, prothrombin time, and creatinine phosphokinase were normal. Inflammatory markers, C-reactive protein and serum ferritin levels, were 4.6 mg/l and 316 ng/ml, respectively. High-resolution computed tomography (HRCT) of chest showed atypical findings – patchy ground-glass opacity in the left upper lobe and evidence of hilar lymphadenopathy. This was assigned a CORADS score of 3 (indeterminate, Coronavirus Disease-2019 Reporting and Data Systems). Other signs such as bilateral ground-glass opacities, consolidation, prominent vessels, or bronchial dilatation were absent. He was diagnosed with mild COVID-19 infection based on HRCT chest findings, D-dimer assay, and level of inflammatory markers. He received dexamethasone and remdesivir along with antibiotic azithromycin. He received medications (amlodipine and telmisartan) for new-onset HTN. Supportive care in the form of incentive spirometry, chest physiotherapy, and humidified oxygen supplementation was also given. His general condition improved within 5–6 days of admission, following which he was discharged.
Examination findings
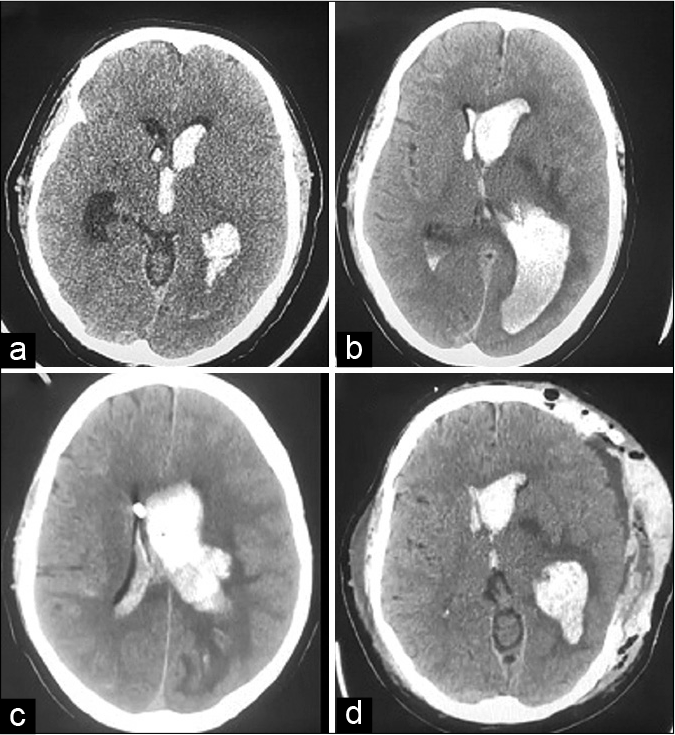
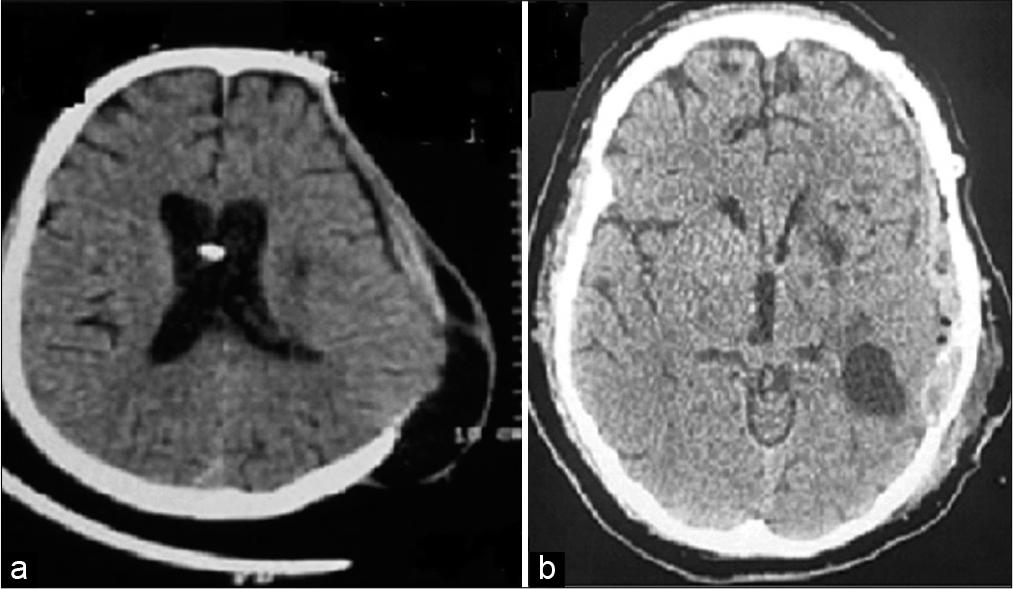
The patient was conscious, alert, and oriented to time, place, and person (Glasgow Coma Scale, [GCS] = E4V5M6) on presentation to the emergency department. Pulse rate was 90/min and blood pressure (BP) 126/80 mmHg. He had right-sided hemiparesis with power <3/5 (MRC grade) on neurological examination. Within an hour of admission, he developed persistent headache and vomiting. Noncontrast computerized tomography of head (NCCT) showed evidence of intraventricular hemorrhage (IVH) in bilateral lateral ventricles, third and fourth ventricles [
Figure 1:
(a) NCCT head showing intraventricular hemorrhage, (b) NCCT head showing intraventricular hemorrhage with ventriculomegaly, (c) NCCT head showing EVD catheter tip inside right lateral ventricle, with reduced ventriculomegaly, and (d) NCCT head showing post decompressive craniectomy status – reduction in midline shift. NCCT: Noncontrast computerized tomography of head, EVD: External ventricular drainage.
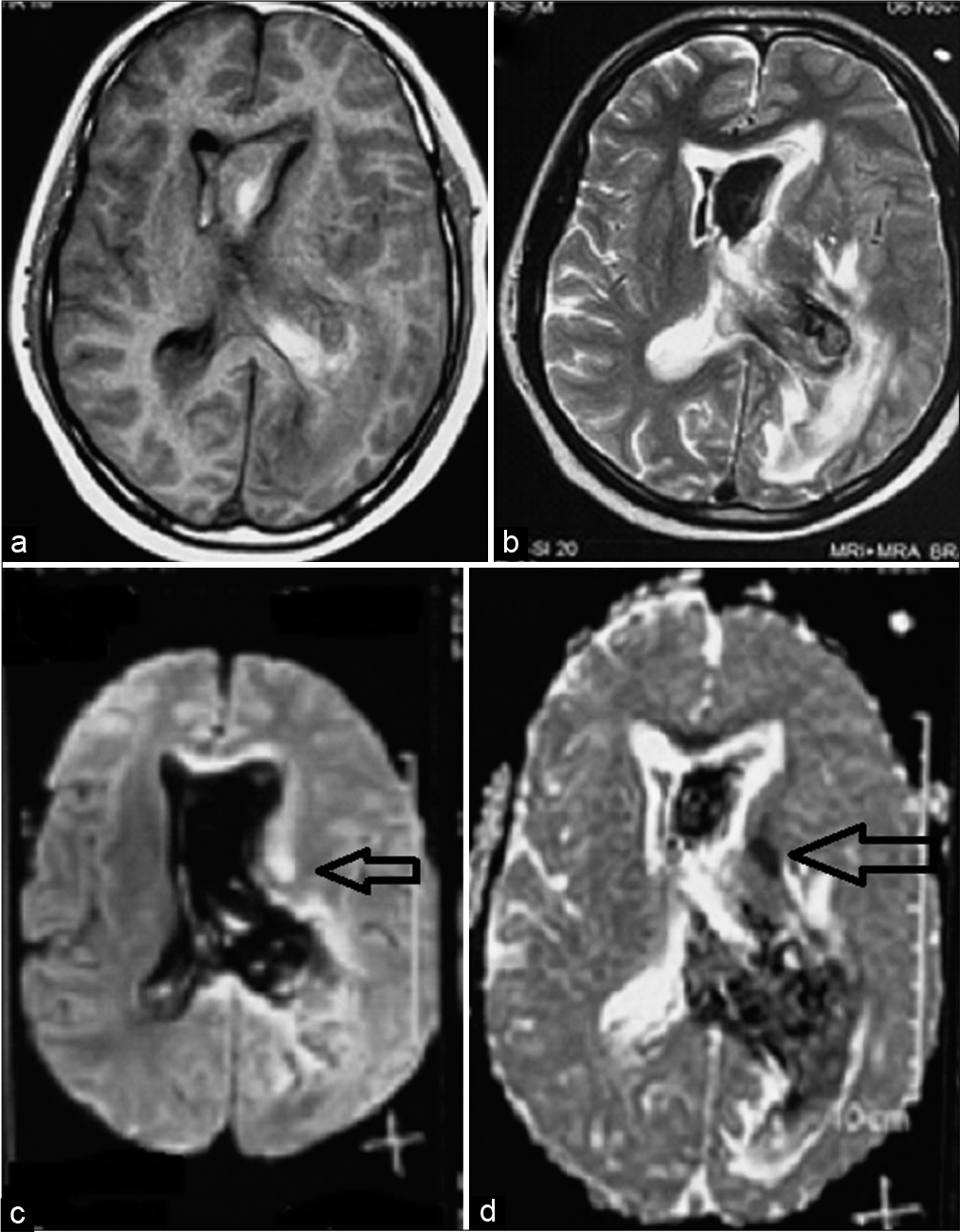
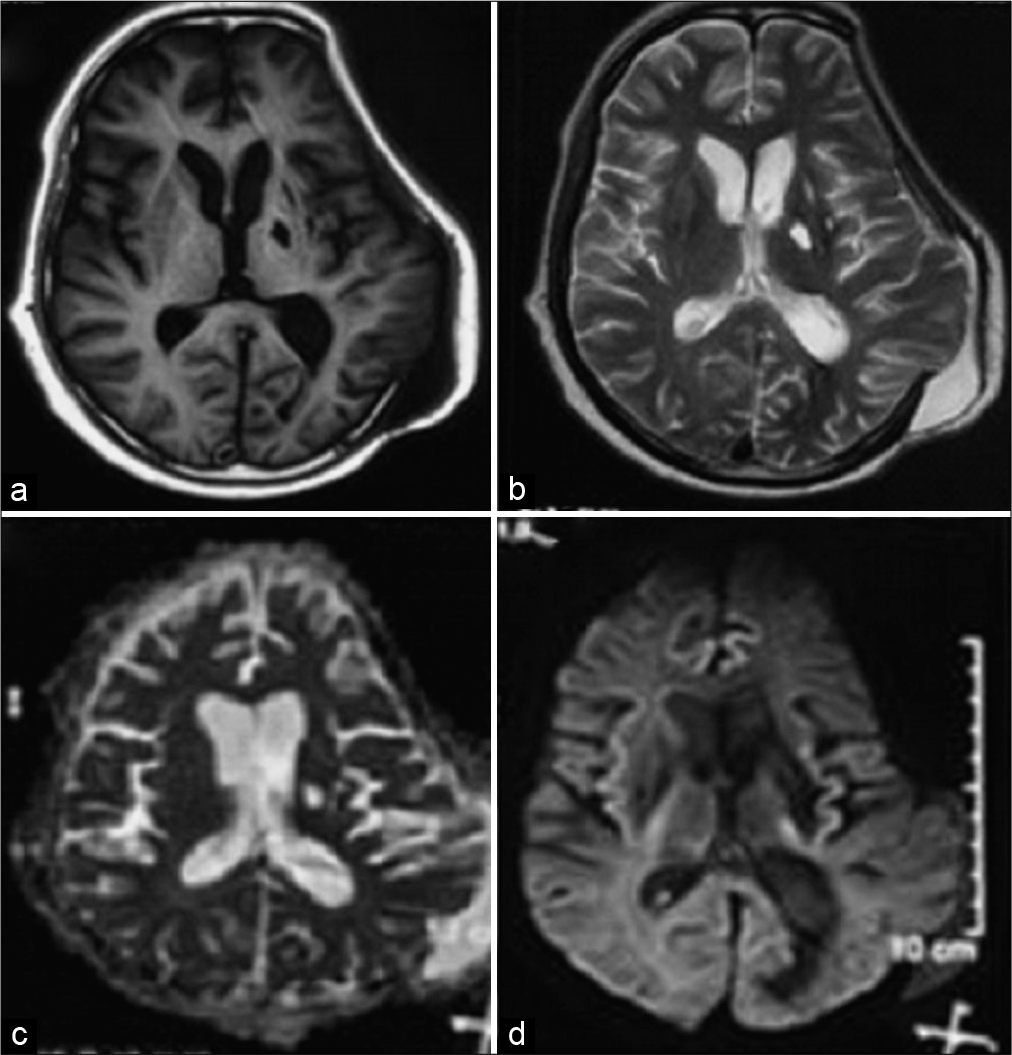
On POD 6, GCS became E2 V3 M5 and right-side hemiparesis worsened. A magnetic resonance imaging brain with angiography was done which was suggestive of an acute infarct in the left internal capsule [
Figure 4:
(a) T1-weighted MR image showing intraventricular hemorrhage, (b) T2-weighted MR image showing intraventricular hemorrhage, (c) diffusion-weighted image showing infarct in the left internal capsule, and (d) apparent diffusion coefficient image showing infarct in the left internal capsule (horizontal black arrow).
On POD 1 after second surgery, his GCS was E2VtM5. Since GCS was lower than preoperative level, mannitol was restarted and periodic CSF drainage was continued from the EVD system. On POD 2, as his GCS improved to E3 VT M6 (occasionally following commands), he was removed of mechanical ventilation. Antibiotic coverage was escalated in view of fever and haziness in the lower zone of the left lung (suggestive of aspiration pneumonia). External CSF drainage system was removed on POD 3, as regular clear CSF flow was noticed and measured pressures were in the range of 10–12 cm H2O. GCS improved to E4 V1 M6 (following command). The left-sided pneumonia gradually improved with antibiotics, chest physiotherapy, nebulized bronchodilators (salbutamol), and mucolytics (N-acetylcysteine). On POD 7, repeat NCCT head showed operative changes with a resolving IVH. Over next few days of hospital stay, there was an improvement in the right upper limb finger movements and flexion movements at the elbow joint.
At the time of discharge, his vitals were stable and GCS was E4V3M6. Pupils were 2 mm bilaterally. He had right-sided weakness −3/5 (MRC grade) in elbow and fingers of upper limb, and 1/5 (MRC grade) in the lower limb. He was discharged with advice to continue antihypertensives and antiepileptics. In view of intracranial hemorrhage, antiplatelets and injectable antithrombotic measures were avoided.
POD 36 (Follow-up)
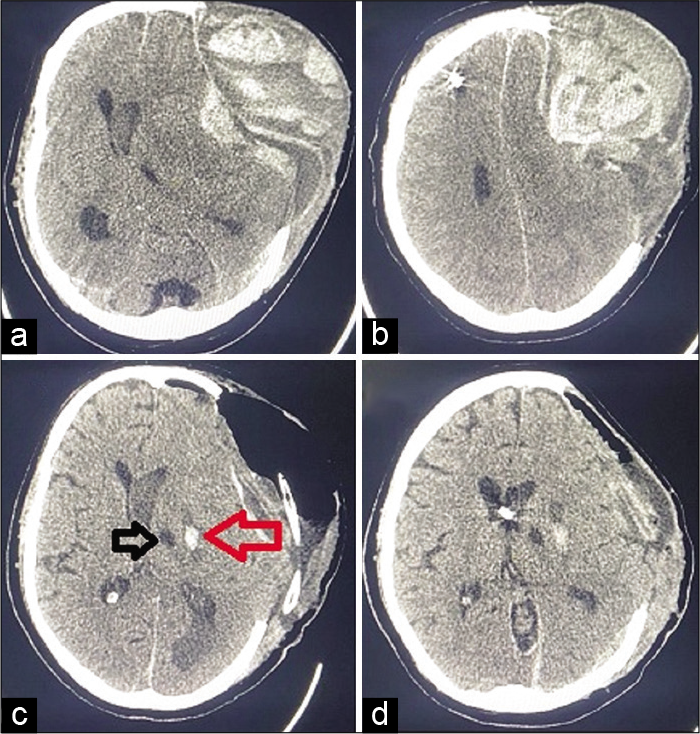
He was brought to ED in an unconscious state. His relatives reported a history of headache and one episode of the right-sided focal seizure followed by unconsciousness, while he was at home. Home recorded BP was 200/100 mmHg, at the onset of headache. On examination, his GCS was E1V1 M2 with extensor posturing. Anisocoria was present with a dilated left-sided pupil. NCCT head showed a large extradural hematoma [
Figure 5:
(a and b) NCCT head at POD 36 showing large extradural hematoma at the site of decompressive craniectomy, (c) postoperative NCCT head showing air in the extradural space after evacuation of hematoma, horizontal black arrow showing gliotic region of old infarct, and horizontal red arrow showing new area of putaminal hemorrhage, and (d) sequential cut image of postoperative NCCT head showing reduced midline shift after hematoma evacuation and old ventricular catheter tip in the right lateral ventricle. NCCT: Noncontrast computerized tomography of head, POD: Postoperative day.
At 6-month follow-up visit, his GCS was E4V1 (Aphasic) M6 with the right hemiparesis (3/5 MRC grade at shoulder, elbow, and wrist joints). MR brain did not reveal any new infarcts [
At 11-month follow-up visit, the patient was E4V1 M6 with residual paresis in the right side (3/5 MRC grade in the upper and lower limbs). MR brain showed a resolving infarct without any new hemorrhagic changes [
DISCUSSION
Several case reports and series have demonstrated the wide nature of COVID-19 infection-related intracranial manifestations. The neurological sequelae include encephalitis, anosmia, stroke, and encephalopathy. Stroke represents one of the most devastating complications.[
Time interval between diagnosis of COVID-19 and occurrence of stroke
Our patient suffered a stroke 4 weeks after the diagnosis of mild COVID-19-associated pneumonia. Available literature shows a duration of 2–25 days separating the two events.[
Use of anticoagulation
It is believed to contribute to the development of hemorrhagic stroke in patients admitted in intensive care. Anticoagulation with low-molecular-weight heparin (LMWH) became a choice of the treatment for COVID-19-infected patients with elevated D-dimer assays. Our patient did not receive LMWH or antiplatelets (aspirin or clopidogrel). Hence, both episodes of hemorrhage were considered noncoagulopathic in nature.
Role of HTN in COVID-19 encephalopathy
Vicenzi et al. demonstrated rise in systolic BP in COVID-19-infected patients with poor pulmonary function reserve. This was seen even in the absence of prior history of HTN.[
Pathophysiology of neurological manifestations
Ischemic stroke has been attributed to COVID-19-related vasculitis, cardiomyopathy, and hypercoagulability.[
Multiple pathogenic mechanisms have been described for hemorrhagic stroke in patients with underlying COVID-19 infection. SARS-CoV-2 has the ability to bind overexpressed endothelial protein angiotensin-converting enzyme 2 (ACE-2) and invade cerebral vasculature. This endotheliitis secondary to the presence of viral inclusion particles is a precursor for rupture of small caliber vessels.[
The above mechanism also reduces serum ACE-2 levels and this unopposed action of angiotensin II, vasopressin, and aldosterone contributes to vasoconstriction and vascular inflammation.[
Another contributing factor to the development of ICH is the cytokine storm resulting from systemic inflammatory syndrome. This cytokine storm has a bimodal mechanism of action. First, the cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha activate matrix metalloproteinases which degrade the elastin and collagen constituents of the extracellular matrix.[
Hemorrhage involving multiple cranial compartments was reported in 14 cases (9.5%). Single compartments were involved in the rest, with intraparenchymal hemorrhage (IPH) being the most common variety (62.6%), followed by subarachnoid hemorrhage (SAH) (15.0%), subdural hemorrhage (SDH) (11.6%), and IVH (1.4%). In patients with IPH, the most common location of the hemorrhage was the cerebral lobes (93.5%). Other sites included basal ganglia (5.4%) and the cerebellum (1.1%).[
Role of inflammatory markers
Ageno et al. demonstrated that D-dimer levels were significantly higher in cardioembolic strokes compared to lacunar strokes.[
Simultaneous occurrence of multiple neurological findings
Multicompartmental hemorrhage has been reported in seven patients out of a large series of COVID-19-infected patients with varied neurological findings.[
Our patient developed spontaneous noncoagulopathic nontraumatic extradural hemorrhage at the site of decompressive craniectomy, 1 month after surgery. On surgical reexploration for the evacuation of hematoma, no active arterial cause could be identified. This presents a new picture of postoperative extradural vascular involvement in a neurosurgical patient, previously infected with SARS-CoV-2. Spontaneous extradural hemorrhage in a patient with sickle cell disease has been described in the literature.[
We believe that the good outcome in this patient can be attributed to the following: Timely intervention in the form of external CSF diversion procedure, good intensive care monitoring, early identification of postoperative complication, that is, spontaneous EDH and rapid decompression in the emergency room, good neurorehabilitation practices, prevention of secondary infection, and complications such as deep vein thrombosis and most importantly the good home care and nutrition by family members.
CONCLUSION
Through this rare case scenario, we present the story of a patient with a history of COVID-19 pneumonia who developed concurrent intracranial infarct and IVH. The occurrence of spontaneous nontraumatic noncoagulopathic extradural hemorrhage at the site of decompressive craniectomy presents an intriguing twist in his tale. These findings cannot be completely explained by existing theories of COVID-19 cerebral vasculopathy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Ageno W, Finazzi S, Steidl L, Biotti MG, Mera V, Melzi d’Eril G. Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. Arch Intern Med. 2002. 162: 2589
2. Altschul DJ, Unda SR, De La Garza Ramos R, Zampolin R, Benton J, Holland R. Hemorrhagic presentations of COVID-19: Risk factors for mortality. Clin Neurol Neurosurg. 2020. 198: 106112
3. Cheruiyot I, Sehmi P, Ominde B, Bundi P, Mislani M, Ngure B. Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol Sci. 2021. 42: 25-33
4. Katz JM, Libman RB, Wang JJ, Sanelli P, Filippi CG, Gribko M. Cerebrovascular complications of COVID-19. Stroke. 2020. 51: e227-31
5. Kotey SN, Dike NO, Nani E, Nyame K. Spontaneous epidural and corpus callosum hemorrhage in sickle cell disease an unusual presentation in a Ghanaian patient. Cureus. 2020. 12: e12292
6. Martin JF, Booth RF, Moncada S. Arterial wall hypoxia following thrombosis of the vasa vasorum is an initial lesion in atherosclerosis. Eur J Clin Investig. 1991. 21: 355-9
7. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020. 94: 55-8
8. Owolabi LF, Raafat A, Enwere OO, Mustapha AF, Adamu B, AlGhamdi M. Hemorrhagic infarctive stroke in COVID-19 patients: Report of two cases and review of the literature. J Community Hosp Intern Med Perspect. 2021. 11: 322-6
9. Reddy ST, Garg T, Shah C, Nascimento FA, Imran R, Kan P. Cerebrovascular disease in patients with COVID-19: A review of the literature and case series. Case Rep Neurol. 2020. 12: 199-209
10. Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: Causative or coincidental?. New Microbes New Infect. 2020. 35: 100669
11. Singh H, Patir R, Vaishya S, Miglani R, Kaur A. External ventricular drain related complications-whether continuous CSF drainage via ommaya reservoir is the answer?. Neurol India. 2020. 68: 458
12. Spence JD, De Freitas GR, Pettigrew LC, Ay H, Liebeskind DS, Kase CS. Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 2020. 49: 451-8
13. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009. 78: 539-52
14. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020. 76: 14-20
15. Vicenzi M, Di Cosola R, Ruscica M, Ratti A, Rota I, Rota F. The liaison between respiratory failure and high blood pressure: Evidence from COVID-19 patients. Eur Respir J. 2020. 56: 2001157
16. Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis. 2020. 36: 101642