- Department of Neurosurgery, University of Illinois Chicago, Neuropsychiatric Institute, Chicago, United States
Correspondence Address:
Fady T. Charbel, Department of Neurosurgery, University of Illinois Chicago, Neuropsychiatric Institute, Chicago, United States.
DOI:10.25259/SNI_699_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tatiana Abou-Mrad, Laura Stone McGuire, Syed I. Khalid, Peter Theiss, Ali Alaraj, Fady T. Charbel. Concurrent meningioma and intracranial aneurysm: Insights from an updated systematic review and a case report. 01-Nov-2024;15:396
How to cite this URL: Tatiana Abou-Mrad, Laura Stone McGuire, Syed I. Khalid, Peter Theiss, Ali Alaraj, Fady T. Charbel. Concurrent meningioma and intracranial aneurysm: Insights from an updated systematic review and a case report. 01-Nov-2024;15:396. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13196
Abstract
Background: The concurrent presentation of meningioma and intracranial aneurysm (IA) poses diagnostic and therapeutic challenges, with no standardized management protocol available. This study aims to address this through an updated systematic review, delineating optimal strategies for managing this dual pathology.
Methods: A systematic review was conducted across PubMed, Web of Science, and Embase databases. Articles were screened independently by two reviewers. Treatment strategies and patient outcomes were comprehensively analyzed to formulate a treatment framework based on several characteristics. In addition, one concurrent meningioma and IA case from our institution was presented.
Results: A total of 69 articles comprising 115 patients were included in the study. The cohort exhibited a female predominance (80%) with a mean age of 56 (±13) years. Meningiomas were primarily localized to the frontotemporal and sellar regions, while aneurysms favored the anterior circulation – notably, 16.5% of cases presented with ruptured aneurysms. Management strategies varied based on the spatial relationship between lesions and aneurysm rupture status. In unruptured cases, 34% underwent a single craniotomy for simultaneous resection of both pathologies, while endovascular intervention was favored when the IA originated from an artery feeding the meningioma (73%). Remarkably, postoperative aneurysm rupture occurred in 33% of cases managed solely through tumor resection (range 0–30 days postop).
Conclusion: This study proposes a comprehensive treatment algorithm to guide neurosurgeons in managing concurrent meningioma and IA cases. By considering individual patient intricacies, the feasibility of simultaneous management, aneurysm rupture risk, and symptomatology, this framework is a valuable tool for clinical decision-making in these complex scenarios.
Keywords: Case report, Intracranial aneurysms, Management, Meningioma, Systematic review
INTRODUCTION
Intracranial meningiomas, recognized as the most prevalent benign tumors within the cranial vault, represent a significant portion of neurosurgical cases, underscoring their clinical importance.[
The coexistence of IAs and brain tumors is not a new phenomenon, and the association between the two remains debated.[
As advancements in diagnostic imaging, surgical techniques, and endovascular interventions have evolved, we sought to offer a clear and comprehensive approach to managing concurrent meningiomas and IA. Building on the foundation laid by De Souza et al., this study aims to bridge the gap in the existing literature by offering an updated systematic review focused on managing patients with this dual pathology.[
METHODS
Literature search
A comprehensive literature search was conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines using three significant databases: PubMed, Embase, and Web of Science. The search strategy was designed to encompass a broad range of terms and their variations to ensure a thorough retrieval of relevant articles. Key search terms included combinations of the following: “(Brain tumor OR Meningioma) AND (aneurysm) AND (coexistent OR simultaneous or concurrent).” To capture the broadest possible range of studies, variations and synonyms for these terms, such as “brain tumor,” “coexist,” and “coexisting”, were also employed. The search was conducted without applying any filters or limitations and included all records up to October 2023.
Study selection
Following the search, all identified records were imported into Covidence, a software tool designed to streamline the management of systematic reviews. Covidence facilitated the automatic removal of duplicate entries, ensuring the efficiency of the screening process. Two independent reviewers (T.AM and L.S.M) meticulously screened the articles. Each article was independently evaluated for relevance, with any remaining duplicates manually removed. Predefined inclusion and exclusion criteria were applied. Only original research articles written in English that detailed the management and outcomes of patients with concurrent meningioma and IAs were included in the study. Exclusion criteria included studies on other tumor types or vascular malformations, articles in languages other than English, those lacking sufficient details on treatment strategies or patient outcomes, and review articles.
Data extraction
Extracted data included patient demographics, tumor characteristics (location and size), aneurysm specifics (number, size, location, rupture status, and anatomical relationship with the tumor), clinical presentation, treatment details (number and type of procedures, order and time interval between interventions), as well as outcomes (intra- and postoperative complications, follow-up duration, and patient status at last follow-up). The aneurysm’s anatomic relationship to the tumor was extracted as described in the paper, distinguishing between feeder artery aneurysms (IA on artery feeding the tumor), embedded aneurysms (IA encased by tumor), proximal aneurysms (IA bordering the tumor, adjacent, or located on an artery close to the meningioma), and distal/remote aneurysms (IA on the artery that is far from the tumor, in a distant ipsilateral location in the brain, or situated in another hemisphere). This classification schema was consistently applied, even when the paper did not explicitly state the anatomical relationship between the IA and meningioma. A visual representation of these anatomic relationships is provided in
Statistical analysis
Descriptive statistics were performed to summarize the findings from the literature review. Given the nature of our study as a systematic review, no comparative statistical analysis was performed. Instead, the goal was to interpret findings across the included studies and formulate a comprehensive treatment framework for managing this dual pathology.
RESULTS
Case presentation
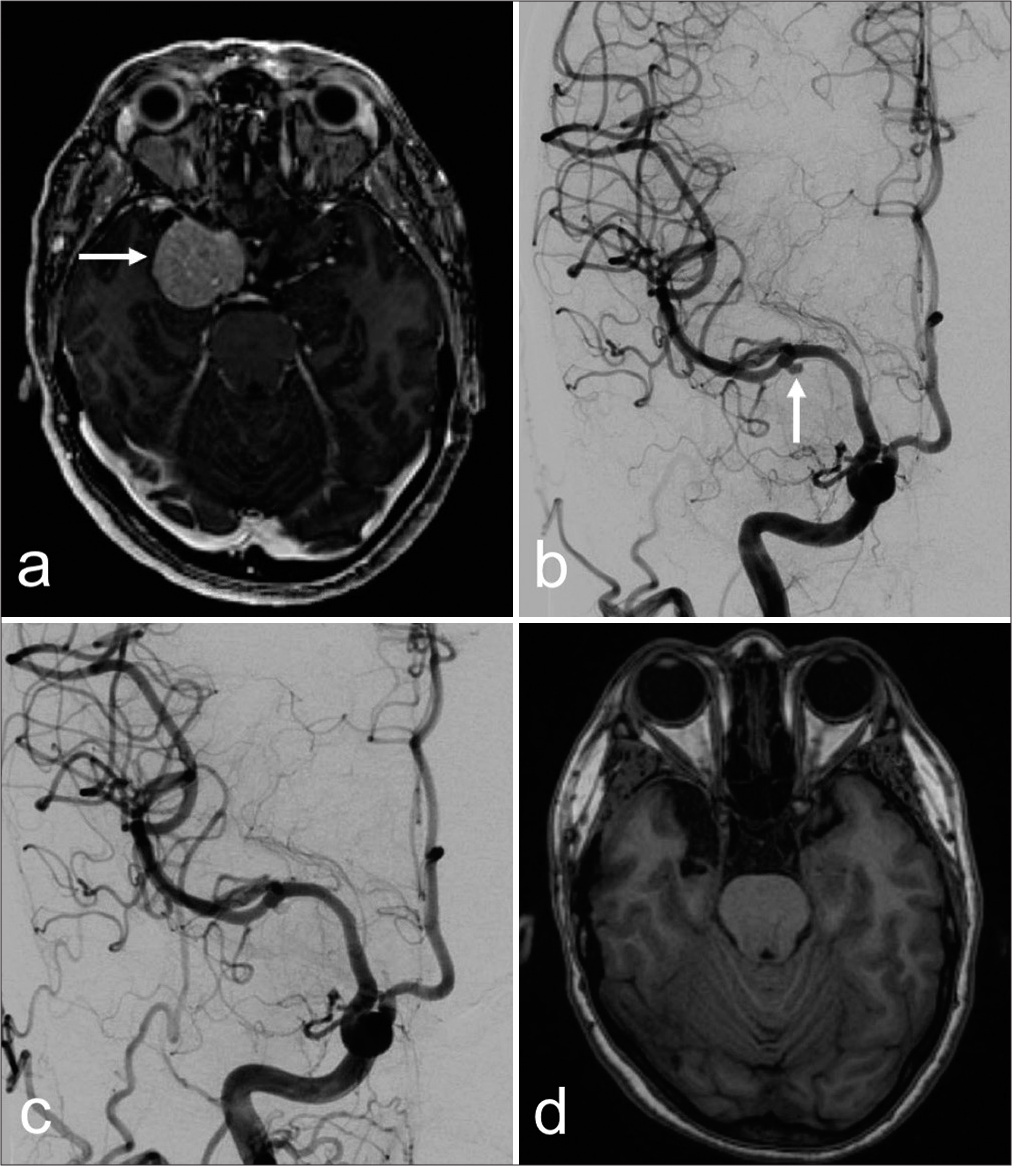
A 54-year-old woman with a history of hypertension was being followed for an asymptomatic right sphenoid wing meningioma. The tumor was found to encase the right supraclinoid and cavernous segments of the internal carotid artery (ICA), along with the proximal M1 segment with extension to the cavernous sinus [
Figure 2:
Axial (a) magnetic resonance imaging (MRI) view depicting a right sphenoid wing meningioma (white arrow) with extension to the cavernous sinus. (b) Digital subtraction angiography view showing an aneurysm at the right middle cerebral artery bifurcation (white arrow). Postoperative angiogram (c) showing resolution of the aneurysm, and (d) MRI showing complete resection of the brain lesion.
Search results
The literature search yielded a total of 1521 articles. After removing duplicates, 1186 articles were screened. A total of 1073 were excluded based on title and abstract screening, leaving 113 articles for full-text assessment. After applying inclusion and exclusion criteria and adding articles identified through reference searches and citation tracking, 69 articles involving 115 patients were included in the study. The article screening and selection process is illustrated in the research flowchart, as shown in
Patients characteristics
The cohort was predominantly female (n = 92, 80%), with an average age of 56 years (±13). The most common presenting symptoms were headache (n = 44, 38%) and visual impairment (n = 32, 27.8%). Nineteen patients presented with a ruptured aneurysm. In addition, a notable subset of patients exhibited multiple pathologies: 10 had more than one meningioma, and 23 had multiple aneurysms. Most meningiomas were supratentorial, with only four infratentorial cases. Meningioma locations ranged from the convexity to the skull base, with the temporal/sphenoid region being the most common site. The majority of aneurysms were located in the anterior circulation (n = 85, 74%), with 14 patients having aneurysms in the posterior circulation, 11 in both anterior and posterior circulations, and 5 involving the middle meningeal artery (MMA). Follow-up durations ranged from 10 days to 14 years. A comprehensive overview of patient characteristics is described in
Meningioma with ruptured aneurysm
Nineteen patients presented with meningioma and concurrent ruptured IA. This subgroup comprised predominantly females (n = 15/19, 79%), with an average age of 56 (±16). The frontal region emerged as the most common meningioma location, with five cases in the frontal convexity and 4 in the clinoidal region. Ruptured IAs were almost exclusively found in the anterior circulation (n = 18/19, 95%), with the anterior communicating artery being the most frequently involved (n = 9/18, 50%). Moreover, most IAs were close to the meningioma, with ten aneurysms being adjacent and six others embedded within the tumor mass. Treatment was administered to 16 out of the 19 patients, with the majority (n = 15/16, 94%) undergoing a single surgical procedure addressing both pathologies simultaneously. Within this subset, the aneurysm was clipped in 11 cases (n = 11/15, 73%). There was one operation-related complication. All the patients who did not receive treatment expired within days of presentation. Further details on these cases are provided in
Meningioma with unruptured aneurysms
Ninety-six cases presented with a co-occurrence of meningioma and unruptured IA. Among them, 11 patients had aneurysms in the feeder artery of the meningioma, seven had aneurysms embedded within the tumor, and the rest involved proximal or distal aneurysms. The anatomical relationship between these two pathologies influenced the feasibility of simultaneous management in most cases and guided the treatment approach.
Eleven cases involved meningiomas with feeder artery aneurysms (FAA) [
Seven cases involved aneurysms embedded within the tumor [
Treatment approaches for the remaining 78 patients varied [
DISCUSSION
The coexistence of IA and brain tumors, particularly meningiomas, is well-documented, though the exact pathological relationship remains elusive.[
Our analysis indicates that IAs frequently occur in proximity to meningiomas, typically in feeder arteries or embedded within the tumor, highlighting the potential impact of hemodynamic stress. Meningiomas, known for their high vascularity, may increase cerebral blood flow, thereby imposing additional stress on arterial walls.[
Vascular assessment in meningioma patients is crucial for identifying concomitant aneurysms, which might otherwise remain undetected until surgery. An intriguing observation from our study revealed that in some cases,[
Previous work by De Souza et al. marked a significant step in understanding the prognosis and survival outcomes for patients with concurrent meningiomas and IAs.[
Patients presenting with ruptured aneurysms alongside meningiomas require immediate intervention, often favoring simultaneous management strategies. Given the vascular nature of meningiomas, differentiating the source of hemorrhage becomes a critical component of the diagnostic and management strategy, especially when both pathologies are nearby. For unruptured aneurysms, treatment decisions hinge on the spatial relationship between the aneurysm and meningioma. A single surgical session can effectively address both conditions when they are in close proximity, minimizing patient exposure to multiple surgeries. However, a staged approach involving preoperative embolization followed by tumor resection also represents a viable option, especially for aneurysms in feeder arteries (73%). Advances in endovascular techniques offer a less invasive alternative that merits consideration based on individual patient criteria. For instance, in our case, although the IA and meningioma were proximal to each other and could have been treated in a single surgery, we opted for a pre-surgical embolization with endovascular coiling given the high-risk nature of the aneurysm to prevent intra-operative rupture. Therefore, for IA with features indicating a high risk of rupture intraoperatively (such as having a daughter sac or a bleb or being tightly adherent to the tumor),[
The variability in management approaches, particularly when aneurysms are distant from the tumor, underscores the need for individualized treatment strategies based on the immediate clinical needs and potential risks. This review has shown that patients treated solely for their tumor had a significant incidence of postoperative aneurysm rupture and mortality (33%), emphasizing the need for careful risk assessment and follow-up. Increased cerebral circulation during surgery due to fluid replacement or changes in blood flow under anesthesia can destabilize the aneurysm wall, making even lower-risk aneurysms more prone to bleeding. Moreover, transient changes in brain architecture due to edema, surgical manipulation, or brain relaxation may alter the tension on the aneurysm or its parent vessel.[
Some authors advocate treating the symptom-causing pathology first.[
Based on clinical expertise and study findings,
Limitations
While our review provides valuable insights into managing concurrent IA and meningiomas, several limitations should be acknowledged. There is a significant publication bias favoring studies with more favorable outcomes or unique clinical scenarios, as this dual pathology is rare and often underreported. The variability among included studies regarding patient population, reporting standards, treatment approaches, and outcomes poses a challenge to drawing definitive conclusions. For instance, the variability in follow-up periods, ranging from 10 days to 14 years, makes it difficult to assess the durability and long-term outcomes of the proposed treatment strategies. The study’s retrospective nature, along with the lack of long-term follow-up data for many cases, especially those managed conservatively, further impacts the generalizability of the results. The absence of comparative statistical analysis is a notable weakness. Although justified by the nature of the systematic review, including some comparative statistics or meta-analysis could have added rigor and depth to the findings. While our review touches on potential pathophysiological links between meningiomas and IAs, it does not delve deeply into the underlying mechanisms, which could provide valuable insights for understanding and managing this dual pathology. Furthermore, the discussion of complications associated with different treatment approaches is brief; a more comprehensive analysis would be beneficial for informing clinical practice. Finally, the proposed algorithm does not incorporate aneurysm size, location, or morphology, as this information was not consistently available in the literature. These factors should be considered, especially when determining an aneurysm’s rupture risk. Moreover, our algorithm lacks full validation, highlighting the need for further refinement and prospective studies.
CONCLUSION
Concurrent IA and meningioma pose a unique challenge requiring a profound understanding of both pathologies and their dynamic interplay. Our proposed algorithm guides treatment by considering the spatial relationship between meningioma and IA, the risk of aneurysm rupture, and patient symptoms. When these pathologies are closely related, simultaneous management might offer a safe and effective treatment route. A staged approach can also be appropriate for some instances, with the decision heavily influenced by specific characteristics of the meningioma and aneurysm. The priority of treatment should be guided by the individual risk of IA rupture and the symptomatic burden of the meningioma. Continuous follow-up and monitoring are crucial, particularly for patients opting for no immediate treatment or managing the non-prioritized pathology in a staged approach. This analysis recognizes the complexity inherent in these situations and highlights the significance of tailoring treatment approaches to each patient’s unique presentation. Future research should focus on prospective studies and standardized guidelines to further refine the proposed management algorithm, including the potential role of radiosurgery in treating small tumors in the cavernous region when an aneurysm is present.
Ethical approval
The research/study approved by the Institutional Review Board at the University of Illinois Chicago, number STUDY2024-0400, dated April 29, 2024.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Algburi HA, Sharma M, Ismail M, Albulaihed SA, Al-Gertani MR, Majeed SN. The coexistence of anterior communicating artery aneurysm and meningioma: A literature review and illustrative case. Surg Neurol Int. 2022. 13: 569
2. Alnaami I, Ho P, Lu JQ, Wheatley B. Case report: Meningioma with intra-tumoural haemorrhage secondary to ruptured distal anterior cerebral artery aneurysm. Open Neuroimage J. 2013. 7: 32-4
3. Arseni C, Maretsis M. Meningioma associated with intracranial aneurysm or cerebral thrombosis. Neurochirurgia (Stuttg). 1973. 16: 131-7
4. Balasubramanian K, Palmisciano P, Scalia G, Crea A, Haider AS, Fagone S. Spontaneous intracerebral pseudoaneurysm rupture and meningiomatosis: A case report and review of the literature. Surg Neurol Int. 2022. 13: 23
5. Bloomgarden GM, Byrne TN, Spencer DD, Heafner MD. Meningioma associated with aneurysm and subarachnoid hemorrhage: Case report and review of the literature. Neurosurgery. 1987. 20: 24-6
6. Carlstrom LP, Peters PA, Van Gompel JJ. Middle meningeal artery aneurysm in a giant meningioma. World Neurosurg. 2023. 170: 65-6
7. Cebral JR, Sheridan M, Putman CM. Hemodynamics and bleb formation in intracranial aneurysms. AJNR Am J Neuroradiol. 2010. 31: 304-10
8. Chen JL, Lee JS, Su ED, Chuang MT, Chen HH. Coexistence of anterior communicating artery aneurysm and tuberculum sellae meningioma. Formosan J Surg. 2015. 48: 226-9
9. Chiriac A, Ion G, Faiyad Z, Poeata I. Associated intracranial lesions: Meningioma and anterior communicating aneurysm. Roman Neurosurg. 2016. 30: 360-4
10. Clarke M. Systematic review of reviews of risk factors for intracranial aneurysms. Neuroradiology. 2008. 50: 653-64
11. Curto L, Squadrito S, Almoto B, Longo M, Granata F, Salpietro F. MRI finding of simultaneous coexistence of growth hormone-secreting pituitary adenoma with intracranial meningioma and carotid artery aneurysms: Report of a case. Pituitary. 2007. 10: 299-305
12. De Bonis C, Gazzeri R, Gorgoglione L, d’Angelo VA. Aneurysm inside meningioma: An unusual association. Br J Neurosurg. 2023. 37: 294-5
13. De Souza MR, Fagundes CF, Rabelo NN, Teixeira MJ, Figueiredo EG. Association between intracranial aneurysm and meningiomas: An integrative survival Analysis with identification of prognostic factors. Clin Neurol Neurosurg. 2020. 198: 106128
14. Delfini R, Domenicucci M, Ferrari M. Association of intracranial meningiomas and aneurysms. Report of three cases and review of the literature. J Neurosurg Sci. 1990. 34: 51-6
15. Ding C, Chen W, Hu Y, Zhang L, Li P. Bilateral aneurysms, one of which is embedded in a meningioma: a rare case report and literature review. Br J Neurosurg. 2021. p. 1-6
16. Dolenc VV, Pregelj R, Slokan S, Skrbec M. Anterior communicating artery aneurysm associated with tuberculum sellae meningioma--case report. Neurol Med Chir (Tokyo). 1998. 38: 485-8
17. Dumitrescu BC, Gorgan MR. Giant tuberculum sellae meningioma with unruptured anterior communicating artery aneurysm encased. Case report and review of the literature. Roman Neurosurg. 2011. 18: 533-40
18. Eulate-Beramendi S, Alvarez-Vega MA, Gutierrez-Morales JC, Lopez-Garcia A. Meningioma associated with brain aneurysm: Report of two cases. Turk Neurosurg. 2017. 27: 321-3
19. Fischer BR, Palkovic S, Holling M, Niederstadt T, Jeibmann A, Wassmann H. Coexistence of cerebral aneurysm and meningioma--pure accident?. Clin Neurol Neurosurg. 2009. 111: 647-54
20. Handa J, Matsuda I, Handa H. Association of brain tumor and intracranial aneurysms. Surg Neurol. 1976. 6: 25-9
21. Hoya K, Yoshimoto Y, Shin M, Nemoto S. Rupture of an internal carotid artery aneurysm within a clinoidal meningioma following stereotactic radiosurgery. Acta Neurochir (Wien). 2011. 153: 1995-6
22. Huntoon K, Toland AM, Dahiya S. Meningioma: A review of clinicopathological and molecular aspects. Front Oncol. 2020. 10: 579599
23. Javalkar V, Guthikonda B, Vannemreddy P, Nanda A. Association of meningioma and intracranial aneurysm: Report of five cases and review of literature. Neurol India. 2009. 57: 772-6
24. Javadpour M, Khan AD, Jenkinson MD, Foy PM, Nahser HC. Cerebral aneurysm associated with an intracranial tumour: Staged endovascular and surgical treatment in two cases. Br J Neurosurg. 2004. 18: 280-4
25. Jimenez JP, Goree JA, Parker JC. An unusual association of multiple meningiomas, intracranial aneurysm, and cerebrovascular atherosclerosis in two young women. Am J Roentgenol Radium Ther Nucl Med. 1971. 112: 281-8
26. Kanamori M, Tomita T, Sasaki T, Murakami K, Takahashi N, Kakehata S. Subarachnoid hemorrhage in a patient with a meningioma and an unruptured aneurysm. Neurol Med Chir (Tokyo). 2013. 53: 343-6
27. Kandel E, Ludkovskaya I, Dobjansky N. Aneurysm inside meningioma. Case report. Acta Neurochir (Wien). 1986. 81: 72-6
28. Kim YH, Lee YJ, Han JH, Ahn S, Lee J, Kim JH. Association of intracranial aneurysms and meningiomas: A case-control study. J Neurosurg. 2015. 123: 357-61
29. Kinali B, Senoglu M, Sandal E, Sandhu G, Karadag A. Management strategy for a patient with coexistence of meningioma and paraophthalmic aneurysm. J Coll Physicians Surg Pak. 2021. 31: 585-7
30. Kuroda H, Takagaki M, Ryuichi H, Yuichi M, Nishida T, Nakamura H. A case of meningolacrimal artery aneurysm associated with meningioma. Surg Neurol Int. 2021. 12: 61
31. Lama M, Mottolese C. Middle meningeal artery aneurysm associated with meningioma. J Neurosurg Sci. 2000. 44: 39-41
32. Lara-Olivas JA, Sangrador-Deitos MV, Villalobos-Díaz R, Marian-Magaña R, Gomez-Amador JL. A rare case of a right infratentorial meningioma and a left giant posterior communicating thrombosed aneurysm. Surg Neurol Int. 2023. 14: 317
33. Lee HS, Park W, Kim YH, Park JC, Ahn J, Hong CK. Coexistence of unruptured anterior communicating artery aneurysm and olfactory groove meningioma in a patient: A case report with a literature review. Surg Neurol Int. 2023. 14: 144
34. Levin P, Gross SW. Meningioma and aneurysm in the same patient. Arch Neurol. 1966. 15: 629-32
35. Licata C, Pasqualin A, Freschini A, Barone G, Da Pian R. Management of associated primary cerebral neoplasms and vascular malformations: 1. Intracranial aneurysms. Acta Neurochir (Wien). 1986. 82: 28-38
36. Ma X, Zhang Y, Zhang C, Yang ZJ, Liu PN. Management principles of cranial base tumor with aneurysm. Neurosurg Rev. 2023. 46: 31
37. Maekawa H, Tanaka M, Hadeishi H. Middle meningeal artery aneurysm associated with meningioma. Acta Neurochir (Wien). 2009. 151: 1167-8
38. Maiuri F, Iaconetta G, Gallicchio B, Sirabella G, Tecame S. Olfactory groove meningioma and multiple aneurysms. Case report. Acta Neurol (Napoli). 1992. 14: 1-5
39. Meguins LC, Hidalgo RC, Spotti AR, de Morais DF. Aneurysm of azygos anterior cerebral artery associated with falcine meningioma: Case report and review of the literature. Surg Neurol Int. 2017. 8: 25
40. Muras I, Rispo A, Bernini FP. Convexity meningioma with an aneurysm of the meningeal vascular pedicle. Riv Neuroradiol. 1999. 12: 585-7
41. Najjar M, Baeesa SS, Lingawi SS. Association between intracranial meningiomas and aneurysms: Is it a coincidence?. Pan Arab J Neurosurg. 2007. 11: 89-97
42. O’Neill OR, Barnwell SL, Silver DJ. Middle meningeal artery aneurysm associated with meningioma: Case report. Neurosurgery. 1995. 36: 396-8
43. Onyia C, Ojo O, Arekhandia B. Sellar brain tumour co-existing with a left posterior communicating aneurysm causing ptosis: Lessons learnt (case report). Pan Afr Med J. 2023. 44: 60
44. Ogino M, Nakatsukasa M, Nakagawa T, Murase I. Ruptured anterior communicating artery aneurysm encased in a tuberculum sellae meningioma. Case report. J Neurosurg. 1999. 91: 871-4
45. Okuyama T, Kurisu K, Hokari M, Miyata K, Uchida K, Asaoka K. Successful treatment with urgent revascularization and parent artery occlusion for a ruptured intratumoral aneurysm following prior meningioma surgery: Illustrative case. J Neurosurg Case Lessons. 2023. 5: CASE23149
46. Papacci F, Pedicelli A, Montano N. The role of preoperative angiography in the management of giant meningiomas associated to vascular malformation. Surg Neurol Int. 2015. 6: 114
47. Papadimitriou K, Rocca A, Dunet V, Daniel RT. Feeding artery aneurysms associated with large meningiomas: Case report and review of the literature. Heliyon. 2020. 6: e04071
48. Paraskevopoulos D, Magras I, Balogiannis I, Polyzoidis K. Anterior clinoidal meningioma coincidental with bilateral intracranial aneurysms. Hippokratia. 2011. 15: 353-5
49. Park KY, Kim BM, Kim DJ. Preoperative coiling of coexisting intracranial aneurysm and subsequent brain tumor surgery. Korean J Radiol. 2016. 17: 931-9
50. Petrecca K, Sirhan D. Paraclinoid aneurysm concealed by sphenoid wing meningioma. Acta Neurochir (Wien). 2009. 151: 171-2
51. Pia HW, Obrador S, Martin JG. Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien). 1972. 27: 189-204
52. Punto L, Fogelholm R, Puranen M, Vapalahti M, Vuolio M. Multiple intracranial meningiomas associated with intracranial arterial aneurysm. Ann Clin Res. 1984. 16: 20-2
53. Raskind R. An intracranial arterial aneurysm associated with a recurrent meningioma. Report of a case. J Neurosurg. 1965. 23: 622-5
54. Scamoni C, Dorizzi A, Dario A, Marra A, Pozzi M. Intracranial meningioma associated with cerebral artery aneurysm. Report of two cases and review of the literature. J Neurosurg Sci. 1997. 41: 273-81
55. Sharma R, Jha R, Bista P. Clinoidal meningioma associated with an anterior communicating artery aneurysm: Case report. Nepal J Neurosci. 2019. 16: 50-3
56. Shibuya M, Takayasu M, Suzuki Y, Sugita K. Coexistence of a laterally projecting C3 carotid aneurysm and a parasellar meningioma. J Clin Neurosci. 1995. 2: 361-4
57. Shigemori M, Tokunaga T, Miyagi J, Eguchi G, Kuramoto S, Irie K. Multiple brain tumors of different cell types with an unruptured cerebral aneurysm--case report. Neurol Med Chir (Tokyo). 1991. 31: 96-9
58. Spallone A, Tcherekayev VA. Simultaneous occurrence of aneurysm and multiple meningioma in Klippel-Trenaunay patients: Case report. Surg Neurol. 1996. 45: 241-4
59. Spitler K, Drazin D, Hanna G, Patel A, Chu R. Association of intracranial aneurysms with meningiomas, pituitary adenomas, and gliomas: Review of possible interrelationships. ISRN Neurol. 2013. 2013: 383425
60. Stevenson JC, Choksey MS, McMahon J, Crawford PJ. Multiple cerebral aneurysms, multiple meningiomas and multiple subcutaneous angiolipomas: A case report. Br J Neurosurg. 1994. 8: 477-81
61. Tachikawa T, Adachi J, Nishikawa R, Matsutani M. An anterior ethmoidal artery aneurysm associated with an olfactory groove meningioma. Case illustration. J Neurosurg. 2002. 97: 1479
62. Takeda N, Nishihara M, Yamanishi S, Kidoguchi K, Hashimoto K. Strategy for patients with co-existence of meningioma and intracerebral aneurysm, especially unruptured aneurysm (-seven cases and review of the literature-). J Clin Neurosci. 2017. 45: 236-42
63. Tanaka S, Kobayashi M, Ichinose T, Oikawa N, Kinoshita M, Yoshikawa A. Intraoperative rupture of intracerebral aneurysm immediately after meningioma resection: A case report. BMC Neurol. 2022. 22: 135
64. Tancioni F, Egitto MG, Tartara F. Aneurysm occurring within a meningioma: Case report. Br J Neurosurg. 1998. 12: 588-91
65. Taylor PE. Delayed postoperative hemorrhage from intracranial aneurysm after craniotomy for tumor. Neurology. 1961. 11: 225-31
66. Suslu HT, Bozbuga M. Primary brain tumors associated with cerebral aneurysm: Report of three cases. Turk Neurosurg. 2011. 21: 216-21
67. Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP. Incidental findings on brain MRI in the general population. N Engl J Med. 2007. 357: 1821-8
68. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
69. von Spreckelsen N, Liebig T, Goldbrunner R, Krischek B. Aneurysms encased in meningiomas: A case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2017. 78: 99-102
70. Waqas M, Hadi YB, Ujjan B, Javed G. Clinoidal meningioma associated with an internal carotid artery aneurysm. BMJ Case Rep. 2015. 2015: bcr2014206707
71. Wei KC, Chang CN, Lin PK. An intracranial aneurysm associated with a recurrent meningioma--a case report. Changgeng Yi Xue Za Zhi. 1994. 17: 284-8
72. Wei RJ, Wu XL, Xia F, Chen JC. Case report and literature review: Treatment of multiple meningiomas combined with multiple unruptured aneurysms in a single operation. Front Surg. 2022. 9: 971068
73. Wu X, Li S, Xie CL, Tang X. Temporal lobe meningioma concurrent with multiple intracranial aneurysms. J Surg Case Rep. 2021. 2021: rjaa581
74. Yang W, Huang J. Meningioma encased ruptured paraophthalmic aneurysm: Case report and review of the literature. Interdisciplin Neurosurg. 2014. 1: 17-20
75. Yildirim AE, Divanlioglu D, Karaoglu D, Cetinalp NE, Belen AD. Pure endoscopic endonasal clipping of an incidental anterior communicating artery aneurysm. J Craniofac Surg. 2015. 26: 1378-81
76. Zhong Z, Sun Y, Lin D, Sun Q, Bian L. Surgical treatment of brain tumor coexisted with intracranial aneurysm--case series and review of the literature. Neurosurg Rev. 2013. 36: 645-56
77. Zhou X, Din Z, Liu H, Li Y. Multiple intracranial aneurysms concurrent with a clinoid meningioma: A case report. Turk Neurosurg. 2024. 34: 358-61
78. Ziyal IM, Aydin Y, Bejjani GK, Kaya AR, Duman H. Multiple meningiomas and intracranial aneurysms: A case report and review of the literature. Acta Neurol Belg. 1998. 98: 221-3