- University of Milano-Bicocca, Milan, Italy,
- Department of Neurosurgery, San Gerardo Hospital, Monza, Italy,
- Department of Neuroradiology, San Gerardo Hospital, Monza, Italy.
DOI:10.25259/SNI_44_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Paola Maria Francesca Cristaldi1,2, Alessandra Polistena1,3, Mirko Patassini3, Camilla de Laurentis1,2, Carlo Giussani1,2, Paolo Remida3. Contrast-induced encephalopathy and permanent neurological deficit: A case report and literature review. 14-Jun-2021;12:273
How to cite this URL: Paola Maria Francesca Cristaldi1,2, Alessandra Polistena1,3, Mirko Patassini3, Camilla de Laurentis1,2, Carlo Giussani1,2, Paolo Remida3. Contrast-induced encephalopathy and permanent neurological deficit: A case report and literature review. 14-Jun-2021;12:273. Available from: https://surgicalneurologyint.com/surgicalint-articles/10894/
Abstract
Background: Contrast-induced encephalopathy (CIE) is a rare condition that occurs after intravenous or intra-arterial contrast agent administration. Patients generally show different ranges of neurological deficits, which generally resolve themselves spontaneously within 24–48 h or in rare cases within 2 weeks.
Case Description: We report a case of CIE in a 54-year-old woman during retreatment for recanalization of communicating anterior artery aneurysm and with no history of allergic reaction to contrast agent. After the procedure, the patient developed right hemiplegia and complete aphasia; an MRI performed at 6 days excluded any signs of new ischemia and revealed a hyperintense signal on FLAIR sequences in the left cortical precentral gyrus corresponding to a hyperintense signal on DWI, suggesting a vasogenic edema. After 6 months, she clinically improved even if her previous neurological status was never restored while radiological findings did not change.
Conclusion: According to the literature, many risk factors may play a role in the pathogenesis of CIE: we hypothesized that, among all of them, chronic hypertension and previous cerebral ischemic lesions were the most important in our case. Further studies are necessary to find the correlation between possible risk factors and neurotoxicity.
Keywords: Brain aneurysm, Cerebral angiography, Contrast agent, Encephalopathy, Neurological deficit
INTRODUCTION
Contrast-induced encephalopathy (CIE) is a rare complication that may occur after intravenous or intra-arterial contrast agent administration. Patients experience a wide range of neurological deficits, often mimicking a postprocedural stroke, which generally resolve themselves spontaneously within 24–48 h or in a few cases 10–15 days. The prognosis is not always favorable: in a limited number of cases, a persistent (>15 days) or permanent neurological impairment and even death have been described. Moreover, only presumable risk factors have been reported.
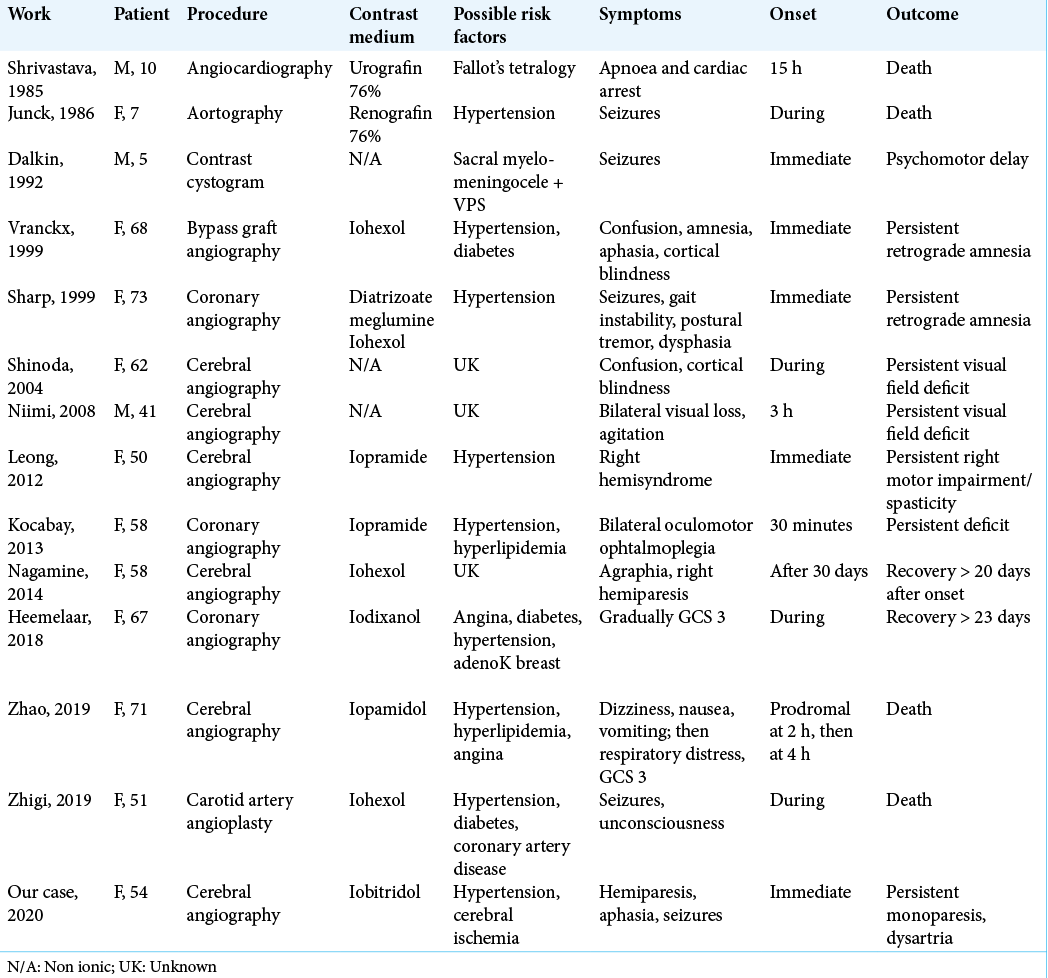
We report the case of a patient with a previous cerebral ischemia and no history of allergic reaction to contrast agent who developed a permanent neurological impairment after contrast medium administration during a cerebral angiography. Furthermore, we update a literature review regarding adverse clinical outcomes following contrast medium exams.
CASE REPORT
A 54-year-old woman was admitted to our hospital in a comatose state. Radiological examinations revealed a subarachnoid hemorrhage from a ruptured aneurysm of the anterior communicating artery. She underwent urgent external ventricular drainage positioning followed by cerebral angiography, resulting in a successful occlusion of the aneurysm with endovascular coiling. After the procedure, she developed a status epilepticus, which was efficaciously treated. A cerebral angiography showed a moderate distal vasospasm and an MRI demonstrated an ischemic area in the left precentral frontal lobe with no clinical correlation. She was discharged and transferred to a rehabilitation hospital.
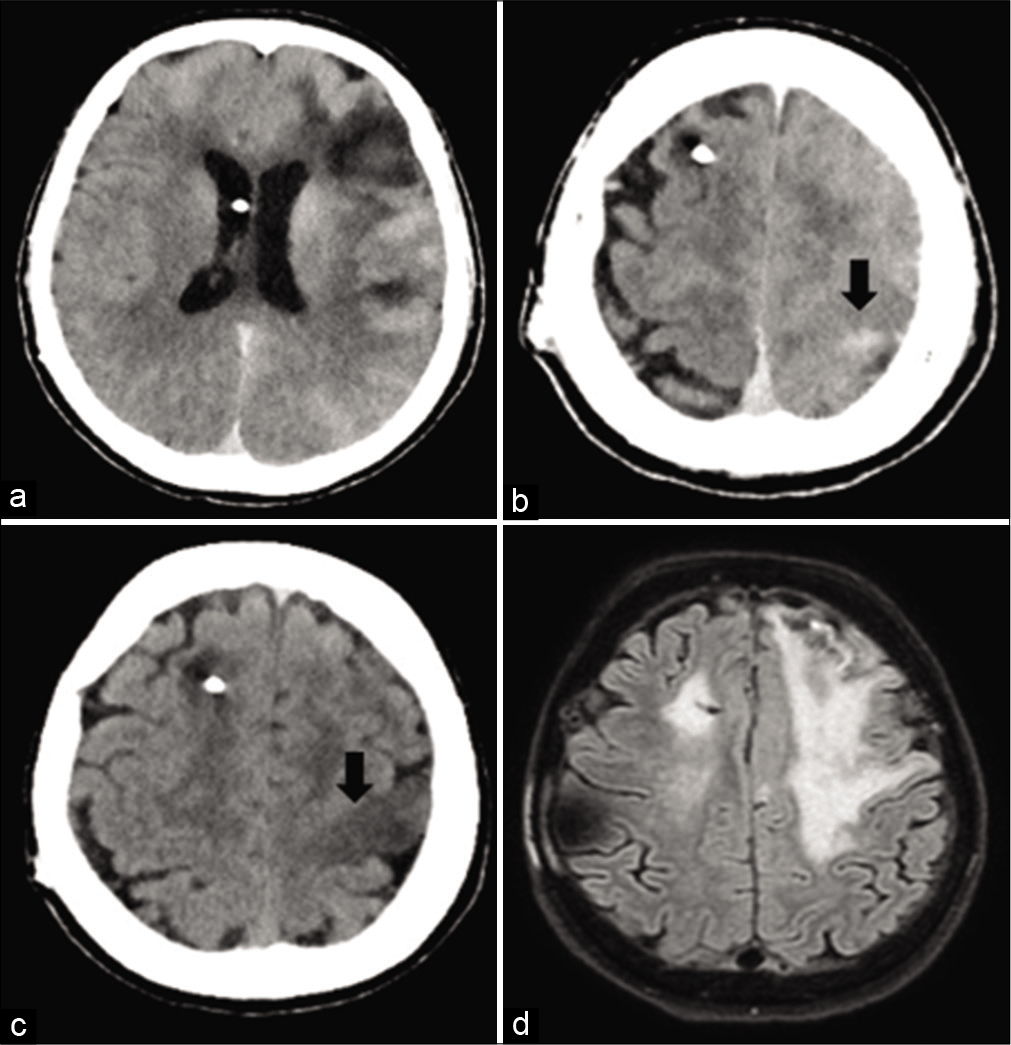
Seven months later, a follow-up cerebral angiography showed a recanalization of the aneurysm, which required retreatment. At that point, she had also developed severe hypertension and was able to suspend antiepileptic treatment. During retreatment, intravenous nimodipine was administered because of ongoing vasospasm throughout the procedure. Endovascular treatment was prolonged due to a complex vascular anatomy: after unsuccessfully attempting to perform a stent-assisted coiling procedure, a coiling-only procedure was carried out, thus resulting in a much larger volume of contrast medium administration. Final sequences documented no vessel occlusion or emboli. The patient emerged from general anesthesia with severe right hemiparesis and complete aphasia. A postoperative CT scan showed an edematous area in the left cerebral hemisphere, an abnormal subarachnoid contrast enhancement zone (probably secondary to contrast extravasation) and a focal left parasagittal frontal hyperdense area [
Figure 1:
(a and b): a postoperative CT scan shows an abnormal subarachnoid contrast enhancement zone and a focal left parasagittal frontal hyperdense area. (c) A CT scan performed at 20 h shows a resorption of the subarachnoid hyperdensity and a new left parasagittal frontal hypodense area. (d) An MRI performed at 6 days reveals a hyperintense signal on FLAIR sequences in the left cortical precentral gyrus (as in case of vasogenic edema), excluding any signs of new ischemia.
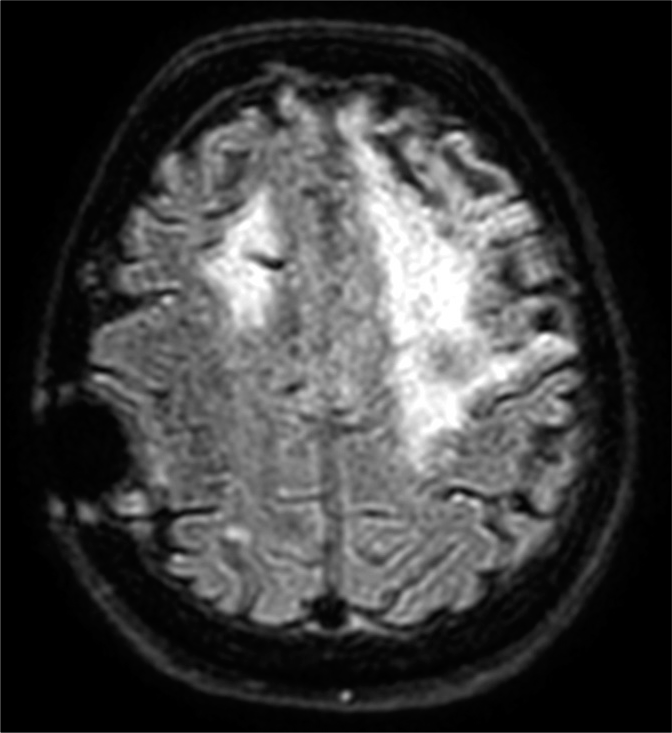
During the following days, her speech and right hemiparesis gradually improved, although she could only compose short sentences and was still not able to walk autonomously. An MRI performed at 6 days revealed a hyperintense signal on FLAIR sequences in the left cortical precentral gyrus (as in case of vasogenic edema), corresponding to a hyperintense signal on DWI sequences with no alteration of the ADC maps, thus excluding any signs of new ischemia [
After 6 months of rehabilitation, the patient improved clinically, being left with a mild dysarthria and a mild right monoparesis, but having regained the capacity to walk and live autonomously.
The patient’s clinical course and radiological findings suggested a possible permanent neurotoxic effect of contrast medium during coil embolization.
DISCUSSION
CIE is a form of neurotoxicity arising from contrast medium. It is mostly described as a transient phenomenon with a favorable prognosis.[
Some possible pathological mechanisms have been proposed. The contrast medium may directly alter the tight junctions between capillary endothelial cells in the brain or may cause a blood displacement and consequently tissue hypoxia. Furthermore, high concentrations of contrast medium may cause clumping of red blood cells and consequently occlusion of arterial branches or even the hyperosmolarity of the contrast medium may cause BBB breakdown.
Selective cortical injury following hypoxic-ischemic insults has been described.[
CONCLUSION
CIE is a form of neurotoxicity arising from contrast media, generally transient but occasionally leading to permanent complications or death. Further studies are necessary to investigate the possible influence of already known risk factors in its pathogenesis and to rule out the presence of new contributing factors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018. 2018: 9278526
2. Frontera JA, Pile-Spellman J, Mohr JP. Contrast-induced neurotoxicity and selective cortical injury. Cerebrovasc Dis. 2007. 24: 148-51
3. Guimaraens L, Vivas E, Fonnegra A, Sola T, Soler L, Balaguer E. Transient encephalopathy from angiographic contrast: A rare complication in neurointerventional procedures. Cardiovasc Intervent Radiol. 2010. 33: 383-8
4. Hamra M, Bakhit Y, Khan M, Moore R. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017. 13: 331-5
5. Kocabay G, Karabay CY. The diagnosis of contrast-induced neurotoxicity. Vascular. 2014. 22: 391-2
6. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol. 2012. 18: 33-41
7. Yu J, Dangas G. Commentary: New insights into the risk factors of contrast-induced encephalopathy. J Endovasc Ther. 2011. 18: 545-6
8. Zhao W, Zhang J, Song Y, Sun L, Zheng M, Yin H. Irreversible fatal contrast-induced encephalopathy: A case report. BMC Neurol. 2019. 19: 46