- Department of Neurosurgery, Acibadem CityClinic University Hospital Tokuda, Sofia, Bulgaria.
- Department of Imaging Diagnostics Acibadem CityClinic University Hospital Tokuda, Sofia, Bulgaria.
- Department of Cardiology, Acibadem CityClinic University Hospital Tokuda, Sofia, Bulgaria.
Correspondence Address:
Toma Spiriev, Department of Neurosurgery, Acibadem City Clinic University Hospital Tokuda, Sofia, Bulgaria.
DOI:10.25259/SNI_1143_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Toma Spiriev1, Lili Laleva1, Nurfet Alioski1, Raicho Dobrikov2, Valeri Gelev3, Milko Milev1, Vladimir Nakov1. Contrast-induced neurotoxicity presented as transient cortical blindness after stent-assisted coiling of a medium-sized unruptured basilar artery aneurysm: A case report and review of the literature. 11-Feb-2022;13:48
How to cite this URL: Toma Spiriev1, Lili Laleva1, Nurfet Alioski1, Raicho Dobrikov2, Valeri Gelev3, Milko Milev1, Vladimir Nakov1. Contrast-induced neurotoxicity presented as transient cortical blindness after stent-assisted coiling of a medium-sized unruptured basilar artery aneurysm: A case report and review of the literature. 11-Feb-2022;13:48. Available from: https://surgicalneurologyint.com/surgicalint-articles/11383/
Abstract
Background: Contrast-induced neurotoxicity is a rare event after endovascular diagnostic procedures or interventions and presents as transient neurological deficit. Herewith, we present a case of reversible complete cortical blindness after uneventful stent-assisted coiling of a medium-sized unruptured basilar artery aneurysm.
Case Description: A 70-year-old woman with a medium-sized 10 mm/6 mm wide neck basilar tip aneurysm was planned for endovascular obliteration of the lesion. The procedure was done under general anesthesia. The contrast agent was iso-osmolar, nonionic. The aneurysm was coiled, and a stent was placed in the left posterior cerebral artery achieving sufficient aneurysm packing. No signs of vessel obliteration were observed during the procedure. On awakening of anesthesia, the patient reported complete visual loss. Ophthalmological examination was normal. The patient was brought back to the angio-suite but there were no signs of parent vessel compromise from the endovascular implants or distal vessel occlusion. An MRI of the brain was done showing no signs of brain ischemia, just mild brain edema in both occipital lobes. Given the results of the radiological studies and clinical presentation, the diagnosis of contrast-induced neurotoxicity was accepted. In 72 h, the patient had complete resolution of the visual loss and was discharged home with no additional neurological worsening.
Conclusion: Contrast-induced neurotoxicity is a rare event that can occur after uneventful endovascular interventions of the brain vessels. Knowledge of this rare complication, after exclusion of all other possible reversible causes, is important for the treatment and prognosis of the patient.
Keywords: Aneurysm, Contrast-induced neurotoxicity, Endovascular coiling, Endovascular stenting, Reversible neurological deficit
INTRODUCTION
In recent years, the endovascular obliteration of intracranial aneurysms and vascular malformations has greatly advanced the management of cerebrovascular lesions as less invasive alternative to surgery.[
CASE DESCRIPTION
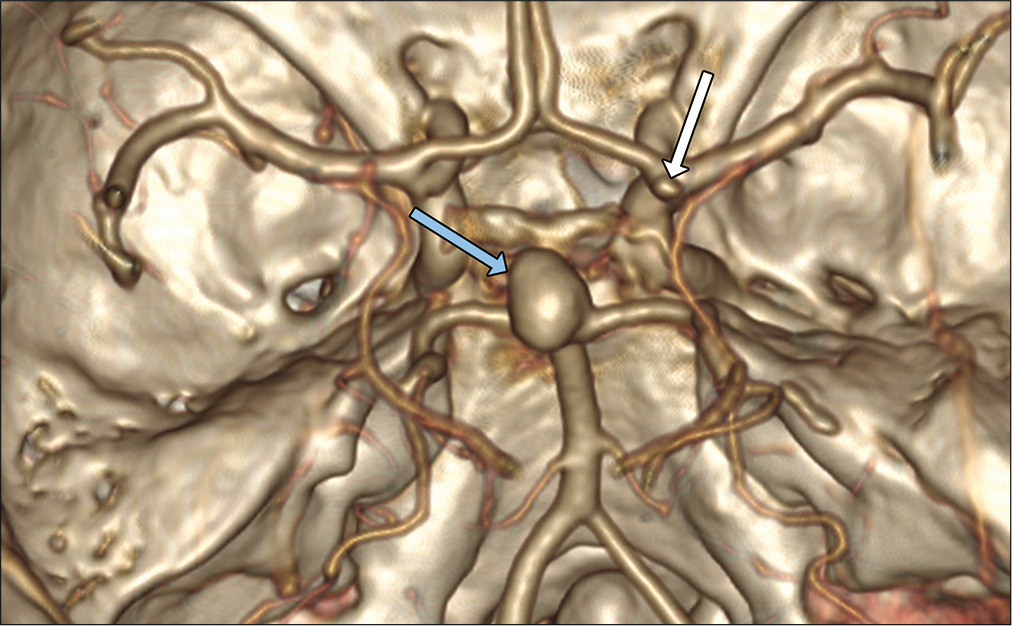
A 70-year-old woman, diagnosed with unruptured mediumsized, 10 mm/6 mm, wide neck (7 mm diameter) basilar tip aneurysm was admitted in our department [
The endovascular obliteration was performed under general anesthesia. The contrast agent was iso-osmolar, nonionic, water soluble. Bilateral femoral artery access was attained with 6F vascular access device on the right side and 5F vascular access device on the left side. A 6F Envoy DA (Codman Neuro®) catheter was introduced in the right vertebral artery and 5F Envoy (Codman Neuro®) catheter was introduced in the left vertebral artery. Using microwire Asahi Chikai® and microcatheter Phenom 27 (Medtronic®), the left posterior cerebral artery (PCA) was catheterized in the preparation of placement of Solitaire (Medtronic®). Another microcatheter Echelon 10 (Medtronic®) was jailed in the fundus of the aneurysm as the Solitaire AB 3 mm/20 mm (Medtronic®) was deployed. The aneurysm was filled with Axium framing coil 12 mm/30 cm (Medtronic®) followed by 7 mm/30 cm and 6 mm/20 cm helical coils achieving sufficient aneurysmal packing. No signs of vasospasm were observed during the procedure [
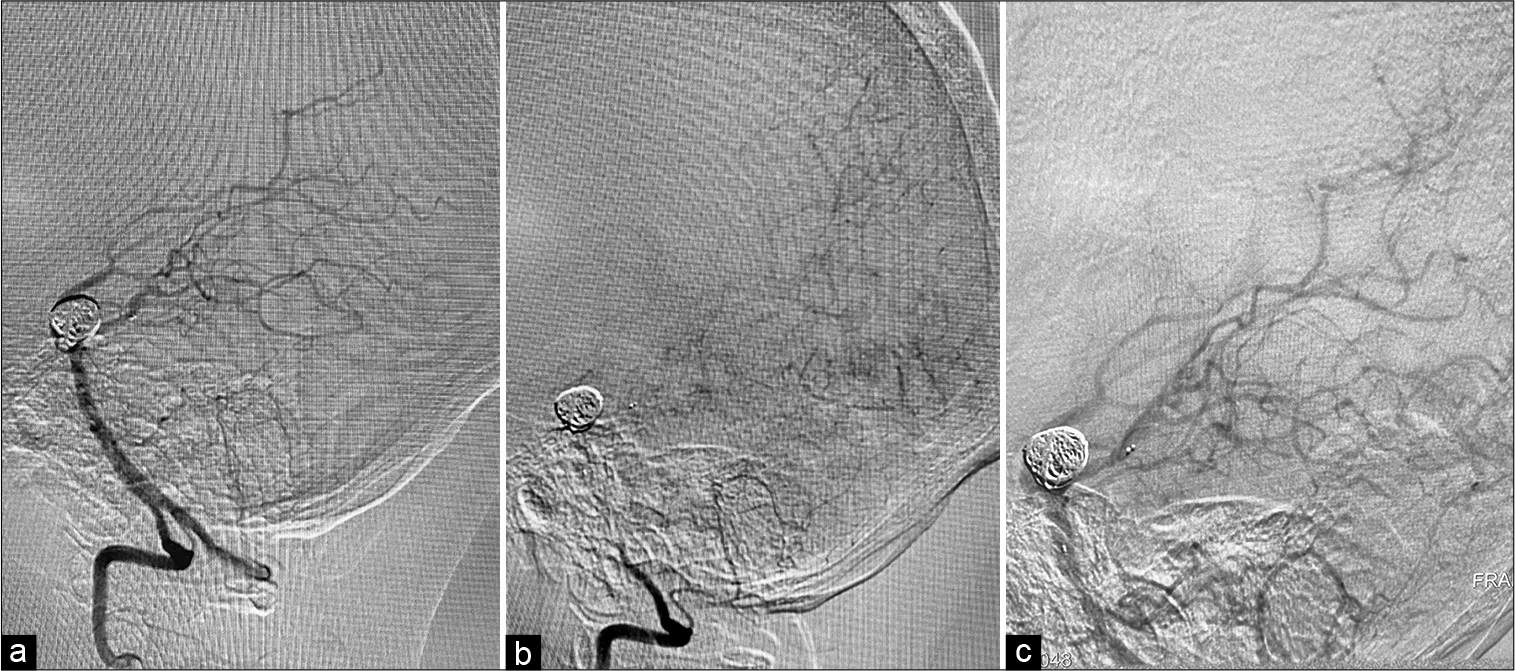
Figure 2:
(a) Intraoperative angiography showing exclusion of the aneurysm from the circulation with coils and stent placement in the right PCA. No vessel compromise is seen, (b) capillary phase of the angiography presenting filling of the small vessels within the occipital lobe, (c) second vertebral angiography after the patient was brought back to the angiography suite presenting good filling of the distal branches of the PCA with no vessel compromise or implant failure.
DISCUSSION
Endovascular neurosurgery, as minimally invasive alternative to microsurgical clipping, is a good option for the treatment of aneurysms in the posterior circulation taking in mind the depth of the vascular lesion and proximity of critical brainstem structures, vessels, and cranial nerves.[
However, endovascular interventions are not without risks and there are various intracranial complications that could also happen throughout the endovascular intervention. These complications include most frequently vasospasm, vessel dissection, parent vessel occlusion, distal emboli, and intravascular device compromise.[
As the condition is very rare, there are not so many studies regarding the risk factors that can precede its occurrence. According to the literature, we suggest grouping the risk factors in three categories: (1) type of endovascular intervention; (2) type of contrast media used during the procedure; and (3) patient-related factors.
Studies suggest that the highest risk for contrast-induced encephalopathy is reported after vertebral angiography, posterior brain circulations studies, and endovascular interventions in this vascular territory.[
The type of contrast agent used during the endovascular procedure is another risk factor. This kind of complication is reported to occur after administration of ionic and nonionic types of contrast agents and might be associated with penetration of the contrast media trough transient disruption of the blood–brain barrier (BBB) related to dose, length of the procedure, and type of the contrast and is predominantly observed in the occipital lobes.[
There are few evidences in the literature for the patient-related risk factors behind this condition. A recent study of Chu et al. examining 421 patients with acute ischemic stroke threated with endovascular thrombectomy report 1.7% (seven patients) who develop transient contrast-induced encephalopathy.[
Several studies regarding the radiological findings observed with this condition describe hyperattenuated lesions in the occipital and posterior parietal cortices visible on CT scan which corresponds to accumulation of contrast media following disruption of the BBB.[
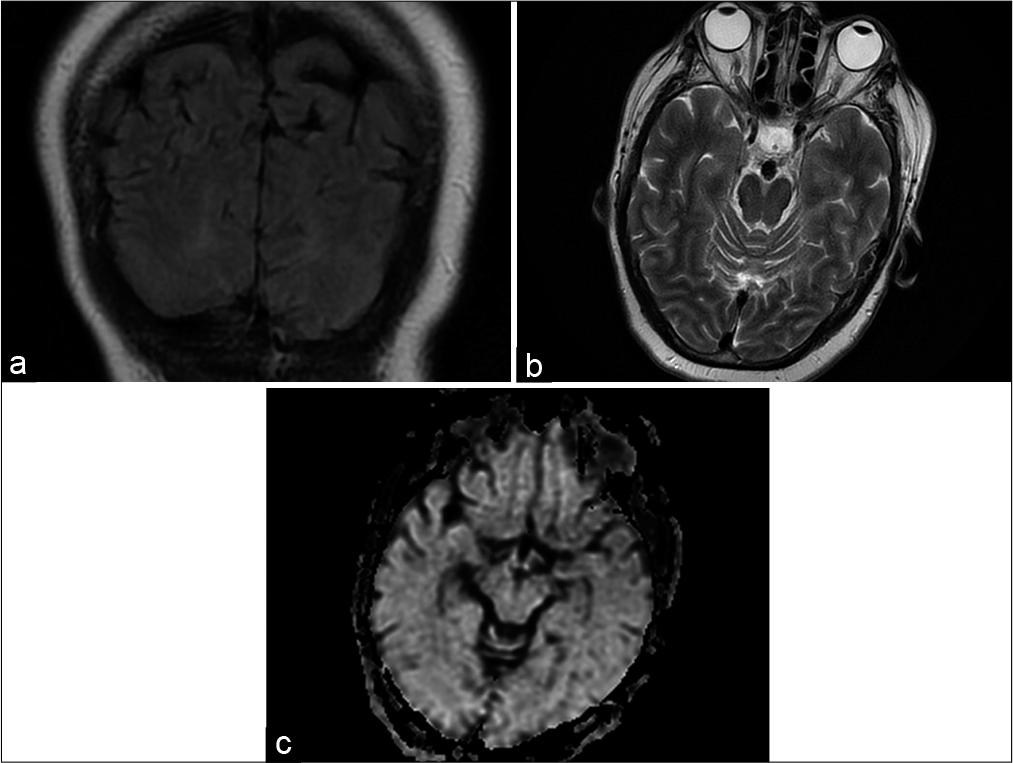
The MRI findings include abnormal cortical T2 and FLAIR hyperintensities in the occipital and parietal cortices, mild brain edema, with the absence of restricted fluid motion on the diffusion-weighted images (DWI), which excludes the ischemic source of the clinical presentation of the disease. In our case we have not done a CT, but an MRI showed bilateral occipital lobe edema only with mild FLAIR changes and no abnormality on the DWI.
In the differential diagnosis with ischemia, which has distinctive MRI features and can be excluded, some studies suggest that contrast-induced neurotoxicity is similar to a syndrome known as posterior reversible leukoencephalopathy with similar MRI characteristics (reversible edema, localized mainly to the occipital lobes). This condition is often related to transplant immunosuppressive treatment, renal insufficiency, and hypertension and is presented with occipital edematous lesions on MRI, which often resolves on control of blood pressure or reduction of immunosuppressive drugs.[
The condition, if all other causes are excluded as in our case, usually requires no special pharmacological treatment and is reversible shortly after the procedure. However, some authors suggest the use of dexamethasone in tapering doses to stabilize the BBB, hydration, or even mannitol to draw contrast media osmotically out of the occipital cortex.[
CONCLUSION
Contrast-induced neurotoxicity is a rare event that can occur after uneventful endovascular interventions of the brain vessels. The condition has benign course, with reversible neurological symptoms. However, patient associated conditions such as impaired renal function, history of previous stroke, intraparenchymal brain lesions, hypertension, as well as interventions in posterior circulation are potential risk factors for the occurrence of contrast-induced neurotoxicity. Although treatment is suggested, there is no evidence for specific therapeutic management. Knowledge of this rare condition, after exclusion of all other possible reversible causes, is important for the prognosis and reassurance of the patient for the temporary nature of the neurological worsening.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bakker NA, Metzemaekers JD, Groen RJ, Mooij JJ, van Dijk JM. International subarachnoid aneurysm trial 2009: Endovascular coiling of ruptured intracranial aneurysms has no significant advantage over neurosurgical clipping. Neurosurgery. 2010. 66: 961-2
2. Bekelis K, Gottlieb DJ, Su Y, O’Malley AJ, Labropoulos N, Goodney P. Comparison of clipping and coiling in elderly patients with unruptured cerebral aneurysms. J Neurosurg. 2017. 126: 811-8
3. Chilingirov N, Zheleva-Kyuchukova I, Stoyanov I, Staykov I, Peneva M, Kirova-Nedyalkova G. Contrast-induced encephalopathy as a rare complication of percutaneous coronary procedures. Med Rev. 2021. 57: 39-43
4. Chu YT, Lee KP, Chen CH, Sung PS, Lin YH, Lee CW. Contrast-induced encephalopathy after endovascular thrombectomy for acute ischemic stroke. Stroke. 2020. 51: 3756-9
5. Darsaut TE, Findlay JM, Magro E, Kotowski M, Roy D, Weill A. Surgical clipping or endovascular coiling for unruptured intracranial aneurysms: A pragmatic randomised trial. J Neurol Neurosurg Psychiatry. 2017. 88: 663-8
6. Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat Rev Neurol. 2016. 12: 699-713
7. Gmeiner M, Gruber A. Current strategies in the treatment of intracranial large and giant aneurysms. Acta Neurochir Suppl. 2021. 132: 19-26
8. Greving JP, Wermer MJ, Brown RD, Morita A, Juvela S, Yonekura M. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014. 13: 59-66
9. Horwitz NH, Wener L. Temporary cortical blindness following angiography. J Neurosurg. 1974. 40: 583-6
10. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material. CT and MRI studies. Neurology. 1989. 39: 567-7
11. Matsubara N, Izumi T, Miyachi S, Ota K, Wakabayashi T. Contrast-induced encephalopathy following embolization of intracranial aneurysms in hemodialysis patients. Neurol Med Chir (Tokyo). 2017. 57: 641-8
12. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005. 366: 809-17
13. Naggara ON, Lecler A, Oppenheim C, Meder JF, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: A systematic review of the literature on safety with emphasis on subgroup analyses. Radiology. 2012. 263: 828-35
14. Naggara ON, White PM, Guilbert F, Roy D, Weill A, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: Systematic review and meta-analysis of the literature on safety and efficacy. Radiology. 2010. 256: 887-97
15. Niimi Y, Kupersmith MJ, Ahmad S, Song J, Berenstein A. Cortical blindness, transient and otherwise, associated with detachable coil embolization of intracranial aneurysms. Am J Neuroradiol. 2008. 29: 603-7
16. Saigal G, Bhatia R, Bhatia S, Wakhloo AK. MR findings of cortical blindness following cerebral angiography: Is this entity related to posterior reversible leukoencephalopathy?. Am J Neuroradiol. 2004. 25: 252-6
17. Schulte-Altedorneburg G, Rüb K, Scheglmann K. Simultaneous ischemic and neurotoxic brain damage after coronary angiography. Neurol Res. 2004. 26: 79-82
18. Shinoda J, Ajimi Y, Yamada M, Onozuka S. Cortical blindness during coil embolization of an unruptured intracranial aneurysm-case report. Neurol Med Chir (Tokyo). 2004. 44: 416-9
19. Sughrue ME, Saloner D, Rayz VL, Lawton MT. Giant intracranial aneurysms: Evolution of management in a contemporary surgical series. Neurosurgery. 2011. 69: 1261-70
20. Utz R, Ekholm SE, Isaac L, Sands M, Fonte D. Local blood-brain barrier penetration following systemic contrast medium administration. A case report and an experimental study. Acta Radiol. 1988. 29: 237-42
21. Zevallos CB, Dandapat S, Ansari S, Farooqui M, QuispeOrozco D, Mendez-Ruiz A. Clinical and imaging features of contrast-induced neurotoxicity after neurointerventional surgery. World Neurosurg. 2020. 142: e316-24