- Department of Neuroscience and Genetics, Albert Einstein College of Medicine, New York City, New York, United States.
Correspondence Address:
Jean M. Hébert, Department of Neuroscience and Genetics, Albert Einstein College of Medicine, New York City, New York, United States.
DOI:10.25259/SNI_1132_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hébert JM. Could an old brain be made young again?. Surg Neurol Int 23-Dec-2022;13:595
How to cite this URL: Hébert JM. Could an old brain be made young again?. Surg Neurol Int 23-Dec-2022;13:595. Available from: https://surgicalneurologyint.com/surgicalint-articles/12067/
As we age, so do our brains. Brain aging, like aging of the rest of the body, is accompanied by complex forms of stochastic damage that occurs to all our classes of macromolecules. Damage occurs to DNA in various forms including base pair mutations, causing loss of information content. DNA damage alone can drive many other features of aging. Damage also accumulates in lipids, including those associated with myelin in the brain, as well as myelin itself, which is very long-lived. And perhaps most damningly, age-associated damage to the extracellular matrix (ECM) alone, like DNA damage, drives many or most of the features of aging.[
This paints a grim picture for our chances of ever reversing aging of the brain (or of any organ for that matter). In fact, if our principle approach is to continue characterizing these complex forms of macromolecular damage so that one day we might address all of them with a dizzying battery of drugs or gene therapies, then brain age reversal has little hope of becoming a reality in the foreseeable future. Drugs are not smart enough to recognize stochastic modifications without disrupting biologically encoded and useful ones. Moreover, the sets of genes that would be required to recognize and reverse the forms of age-related stochastic damage have yet-to-be invented, not to mention the means of somehow delivering these new sets of genes to most cells of the body and brain without causing more harm than good.
PROGRESSIVE BRAIN REPLACEMENT: FOUNDATIONS
The solution, both for the body and the brain, is replacing old tissues and organs with pristine damage-free new ones. Although many groups are developing lab-made organs and body parts (many of which are being used in people already), an obvious question is whether the idea of replacement can apply to the brain. The answer appears to be yes. Two established principles in neurobiology suggest that progressively replacing brain tissue over time will be possible without a discontinuity of function or self.
Plasticity
The first principle is plasticity, which is particularly evident for the neocortex, the part of the brain that encodes our highest cognitive functions, long-term declarative memories, self-identity, and consciousness. Plasticity evolved so that we can learn new things all the time, allowing us to adapt to our ever changing world. Although cortical plasticity has been documented across mammalian species for most or all cortical functions, the most illustrative examples for our discussion can be found in humans of advanced age. If, for example, the eloquent area of the neocortex is destroyed over the course of a few years due to a benign glioma (in contrast to being suddenly destroyed due to a stroke for example), then the individual never loses the ability to speak as language is seamlessly and progressively re-encoded in other areas of the neocortex while the tumor grows.[
Brain precursor cells
This brings up the second established principle in neurobiology that suggest brain age reversal might be possible. Brain precursor cells are programmed to generate a brain (without us knowing how they do it). Our brains develop from a simple sheet of embryonic neuroepithelial cells that are innately programmed to generate normal brain tissue – in all its complexity, with normal local and long-distance wiring. Even when transplanted into the adult neocortex, neocortical precursor cells differentiate and physiologically integrate remarkably well with the surrounding host brain tissue.[
There are three options for a source of this tissue:
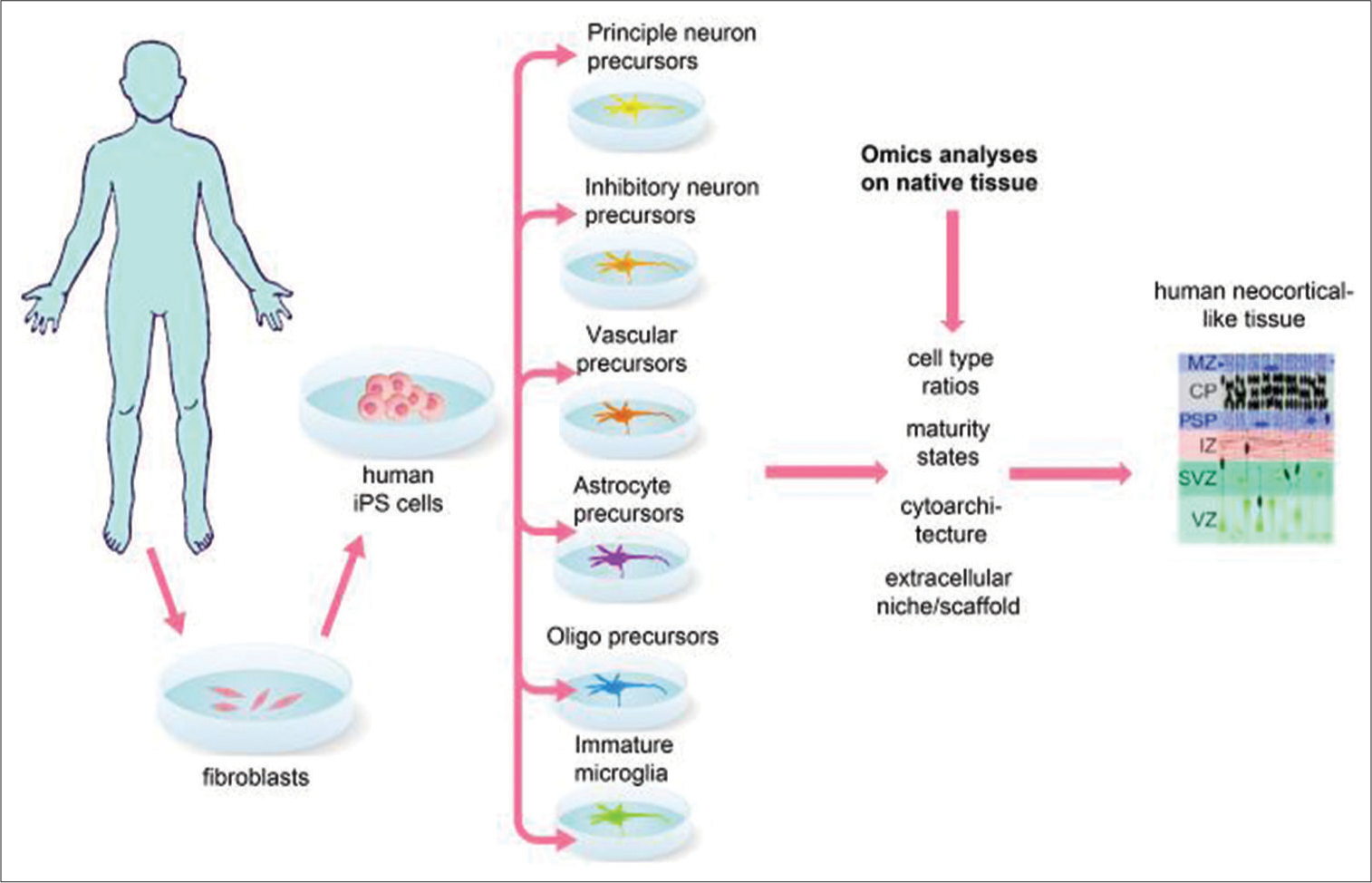
Fetal tissue from pregnancy terminations (as was used for Parkinson’s patients). Although fetal tissue is useful for research and proof-of-concept experiments, it is difficult to scale and would require continued immune suppression of the recipient patient after transplantation. Until therapeutic cloning materializes, which would obfuscate the need for immune suppression, we can put this option aside for now. Laboratory grown or “synthetic” fetuses are under development that could be more easily scaled for widespread use and could be patient derived to avoid immune rejection.[ Reverse engineering of fetal-like brain tissue from human patient-derived iPS cells, which would avoid immune suppression and be scalable. Therefore, we will focus on this option from here on out. Although all brain structures would need to be replaced, let’s first focus on the neocortex, the largest and arguably most important part of the human brain.
PROGRESSIVE BRAIN REPLACEMENT: PROPOSED TECHNIQUE
Step 1: Determine which fetal tissue stage would be best to reverse engineer
There are at least two considerations in identifying an optimal fetal stage for engraftments. The tissue must be young enough for the neurons that it generates to integrate with the host, but the tissue must also have enough structural integrity to maintain a normal layered cytoarchitecture (even the fetal neocortex is comprised of layers, for example, the ventricular zone, subventricular zones, subplate, nascent cortical plate, marginal zone, and pia, all of which play essential developmental roles in generating normal mature tissue). Initial testing can be performed using real fetal neocortical tissue obtained from pregnancy terminations because such tissue has all the precursor cell types with normal ratios, relative differentiation states, and cytoarchitecture. The tissues can be tested by transplanting into aspiration lesions to control the shape and size of the space needed for engraftment. Output measures of graft performance are those routinely used by laboratories, including ours.[
Step 2: Once the optimal fetal tissue stage is determined, identify in detail the components of that tissue
A high-resolution picture of what comprises the stage selected fetal tissue can be obtained using single cell sequencing, which reveals the cell types present and their maturity states,[
The gelling properties of layer-specific scaffolds can be optimized ex vivo, followed by in vivo testing for their ability with the identified growth factors to support vascularization and neuronal differentiation and integration with the host. Our laboratory has recently developed a platform for testing layered, vascularized, multicell type neocortical tissue prototypes in the adult mouse neocortex, which we have initially validated with transplanted mouse rather than human cells and using a commercially available, nonclinically relevant scaffold.[
Step 3: Rebuild neocortical tissue at the site of aspiration lesions in the adult cortex of preclinical animal models
What facilitates the task of engineering human fetal neocortical tissue is that protocols already exist for generating each major fetal neocortical cell type from human iPS cells. For example, precursor cells for excitatory and inhibitory neurons, astrocytes, oligodendrocytes, microglia, and vascular endothelial cells can all be generated and purified from human iPS cells. These precursors could then be reassembled in normal ratios, maturity states, and layered architecture embedded in layer appropriate extracellular niches [
Analyses of graft development and performance would be as in Step 1 and again include characterizing cell survival, mature cell type ratios, tissue organization, axonal and dendritic connections to and from the graft, and functional measures such as responsiveness to visual stimuli when in the visual cortex or ability to elicit movement when in the motor cortex. In addition, a possible experiment to demonstrate that the new tissue encodes part of a useful behavior to the host is to replace a functionally defined host area with new tissue designed so that it can be transiently silenced chemogenetically or optogenetically.
Step 4: Developing the means for progressive silencing and removal of old tissue
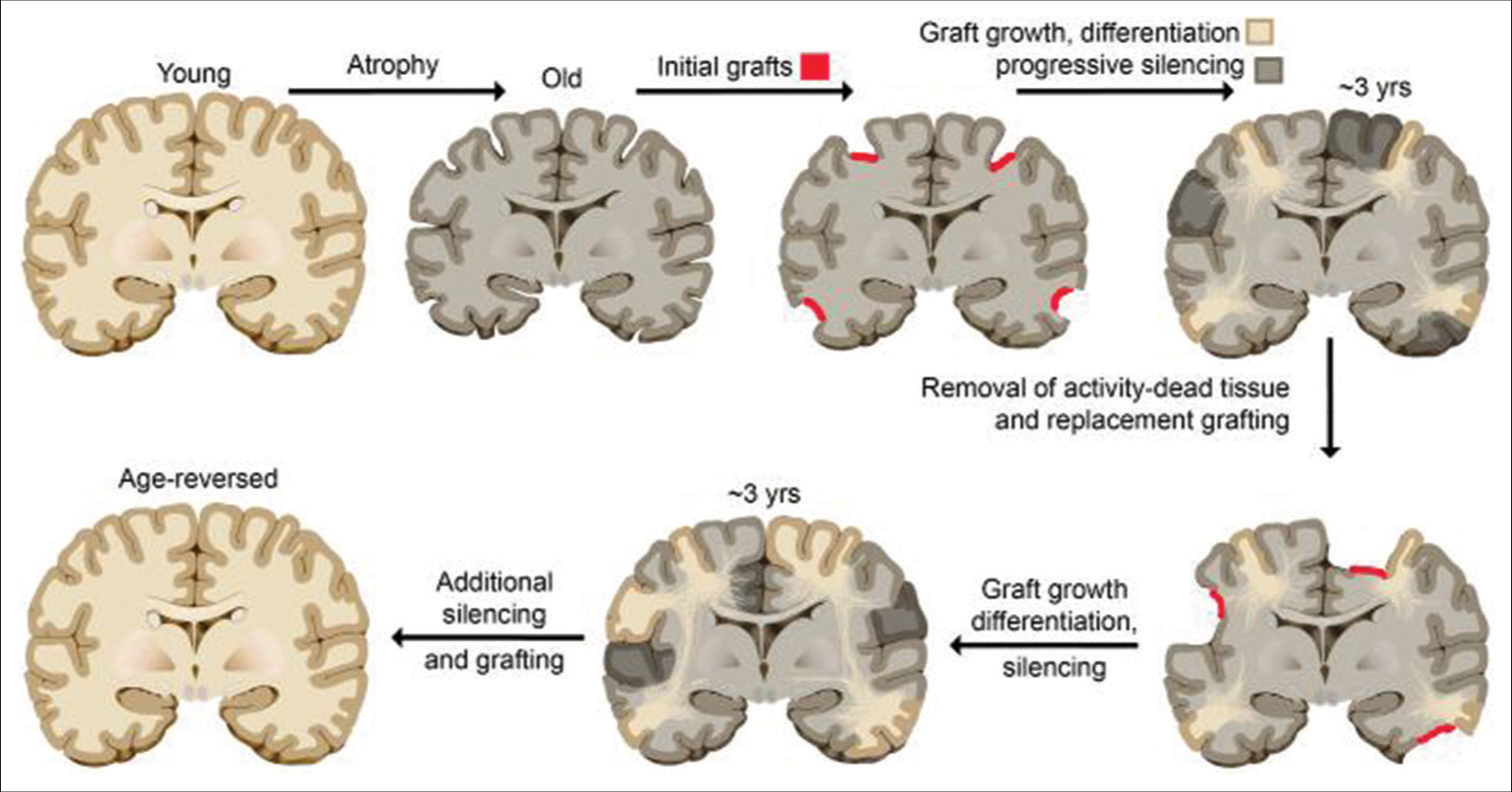
To reverse aging of the brain, progressively adding young tissue is not sufficient; the old tissue must also be progressively removed. As shown in humans of advanced age with slow-growing benign gliomas (described above), if cortical functions are being used while the tissue encoding them is progressively destroyed or silenced, these functions become seamlessly encoded elsewhere. The same idea would apply to intentional silencing of a functional area over time, but without the use of tumors. For example, progressive silencing from a pinpoint location outward could be achieved by infecting the tissue with one of several available red light-shifted opto-silencing channels and progressively increasing the diameter of the laser light used for silencing over time. Once an area of tissue is silenced, then it could be removed without loss of information or function to the individual (as with the resection of slow growing benign gliomas), creating space for new young tissue. These steps could in theory be repeated to revert the entire brain from old to young over the course of a couple of decades without interruption of function or discontinuity of self [
Step 5: Implementation in humans
Once engineered tissue is shown to encode useful information to its hosts in preclinical studies, then it will be ready to test in humans. The first clinical indication would not be brain aging, but instead insults such as stroke or trauma with local loss or degeneration of functional tissue. In this case, the information originally encoded in the tissue would be lost due to the suddenness of the injury, but new immature tissue would be expected by virtue of its extreme plasticity to help relearn the lost functions. Moving forward, damage to greater areas could be addressed, for instance in frontotemporal dementia, and finally, age-related degeneration using the more stepwise approach illustrated in
In sum, despite the massive amount of work needed to achieve engineered fetal-like brain tissue that is fit for progressive replacement in humans, the challenges, which are technical in nature, may not require as much innovation as empirical testing. Hence, brain age reversal could potentially be achieved sooner than we expect, depending on the effort allocated to the task at hand.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aguilera-Castrejon A, Oldak B, Shani T, Ghanem N, Itzkovich C, Slomovich S. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature. 2021. 593: 119-24
2. Amadei G, Handford CE, Qiu C, De Jonghe J, Greenfeld H, Tran M. Embryo model completes gastrulation to neurulation and organogenesis. Nature. 2022. 610: 14353
3. Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: A new door to brain plasticity. Brain. 2007. 130: 898-914
4. Duffau H. The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex. 2014. 58: 325-37
5. Espuny-Camacho I, Michelsen KA, Linaro D, Bilheu A, Acosta-Verdugo S, Herpoel A. Human pluripotent stem-cell-derived cortical neurons integrate functionally into the lesioned adult murine visual cortex in an area-specific way. Cell Reports. 2018. 23: 2732-43
6. Falkner S, Grade S, Dimou L, Conzelmann KK, Bonhoeffer T, Götz M. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature. 2016. 539: 248-53
7. Fedintsev A, Moskalev A. Stochastic non-enzymatic modification of long-lived macromolecules-A missing hallmark of aging. Ageing Res Rev. 2020. 62: 101097
8. Krzyspiak J, Yan J, Ghosh HS, Galinski B, Lituma BJ, Alvina K. Donor-derived vasculature is required to support neocortical cell grafts after stroke. Stem Cell Res. 2021. 59: 102642
9. Magalhaes RS, Williams JK, Yoo KW, Yoo JJ, Atala A. A tissue-engineered uterus supports live births in rabbits. Nat Biotechnol. 2020. 38: 1280-7
10. Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018. 36: 432-41
11. Michelsen KA, Acosta-Verdugo S, Benoit-Marand M, Espuny-Camacho I, Gaspard N, Saha B. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron. 2015. 85: 982-97
12. Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science. 2017. 358: 1318-23
13. Palma-Tortosa S, Tornero D, Hansen MG, Monni E, Hajy M, Kartsivadze S. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc Natl Acad Sci USA. 2020. 117: 9094-100
14. Partridge E, Davey M, Hornick M, McGovern PE, Mejaddam AY, Vrecenak JD. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun. 2017. 8: 15112
15. Quezada A, Ward C, Bader E, Zolotavin P, Altun E, Hong S, editors. An in vivo Platform for Rebuilding Functional Neocortical Tissue. BioRxiv. 2022. p. Available from: https://www.biorxiv.org/content/10.1101/2022.12.09.519776v1 [Last accessed on 2022 Dec 11]
16. Tornero D, Tsupykov O, Granmo M, Rodriguez C, Grønning-Hansen M, Thelin J. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017. 140: 692-706






Marvin Salgado Pérez
Posted January 3, 2023, 10:13 am
EL REEMPLAZO DE NEURONAS CON MALFUNCIONAMIENTO POR NEURONAS APTAS PARA EL FUNCIONAMIENTO NORMAL DEL CEREBRO SERÁ UN SALTO CUÁNTICO EN LA INVESTIGACIÓN NEUROLÓGICA. EN TEORÍA,OPINO QUE ESTAS INVESTIGACIONES IRÁN MAS ALLÁ DE UN EFECTO SCHRÖDINGER .VEREMOS EN EL FUTURO ,NO UN GATO VIVO O MUERTOSI NO,UN GATO VIVO EN CADA NEURONA REEMPLAZADA. DR.MARVIN FERMÍN SALGADO PÉREZ,NICARAGUA,CENTROAMÉRICA.
Moises Vasquez-Loayza.
Posted January 12, 2023, 8:30 am
What impressive work !. All the technological aspects of transplantation seem to have been overcome; but I have a crucial distrust regarding the possibility of connecting the spinal cord and that it works. If this wonder were achieved, it would be to begin the solution to resolve quadriplegic lesions.
Sincerely.