- Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, Utah 84132, USA
- Department of Neurosurgery, Loma Linda University, Loma Linda, California 92354, USA
Correspondence Address:
William T. Couldwell
Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, Utah 84132, USA
DOI:10.4103/2152-7806.170464
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sivakumar W, Oh N, Cutler A, Colman H, Couldwell WT. Cranial and spinal leptomeningeal dissemination in esthesioneuroblastoma: Two reports of distant central nervous system metastasis and rationale for treatment. Surg Neurol Int 25-Nov-2015;6:
How to cite this URL: Sivakumar W, Oh N, Cutler A, Colman H, Couldwell WT. Cranial and spinal leptomeningeal dissemination in esthesioneuroblastoma: Two reports of distant central nervous system metastasis and rationale for treatment. Surg Neurol Int 25-Nov-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/cranial-and-spinal-leptomeningeal-dissemination-in/
Abstract
Background:Esthesioneuroblastoma is a locally aggressive cancer of the nasal cavity. While systemic metastasis can occur in 10-30% of patients, there are only six reported cases of distal metastasis from leptomeningeal dissemination.
Case Description:The authors report two cases of esthesioneuroblastoma treated previously with multimodal therapy in which distal metastatic recurrence was found and describe their treatment protocol, which has resulted in long-term success.
Conclusion:Understanding the drivers of leptomeningeal dissemination in more prevalent primary neuroectodermal tumors may hold the key to developing successful treatment algorithms for this disease.
Keywords: Carcinomatosis, chemotherapy, esthesioneuroblastoma, leptomeningeal dissemination, olfactory neuroblastoma, primary neuroectodermal tumor, radiation, surgery
INTRODUCTION
Esthesioneuroblastoma, or olfactory neuroblastoma, is a locally aggressive cancer of the nasal cavity.[
Systemic metastases occur in 10–30% of patients, usually by hematogenous and lymphatic spread,[
CASE REPORTS
Patient 1
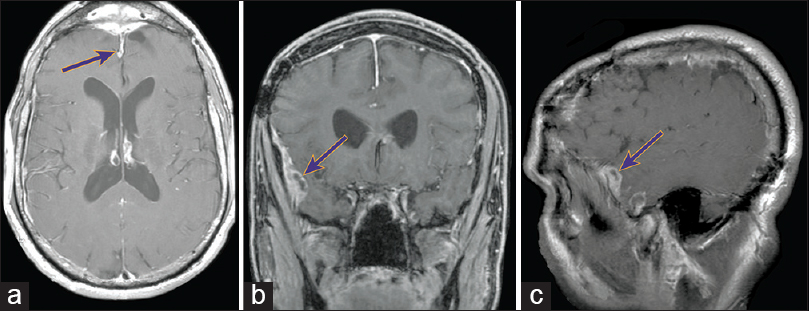
A 48-year-old man was referred to our clinic for evaluation of a new dural-based lesion 6 years after excision, skin flap repair, and bone graft placement for a skull-base esthesioneuroblastoma followed by adjuvant radiation. His posttreatment course was complicated by externalization of the bone flap requiring a second skull-base bone flap resection. After 5 years of no radiographic evidence of residual disease, a routine follow-up magnetic resonance imaging (MRI) scan at 6 years revealed an enhancing dural lesion over the right frontal lobes [
The patient underwent a right frontotemporal craniotomy for resection of the enhancing dura and tumor, which was found to be partially invading the brain parenchyma. Pathological analysis indicated the lesion was consistent with recurrent metastatic esthesioneuroblastoma. The patient received adjuvant stereotactic radiosurgery. His follow-up MRIs approximately every 6 months remained negative until additional dural-based lesions along the frontal sinus, right temporal lobe, and right sphenoid wing were observed at 3 years [
Patient 2
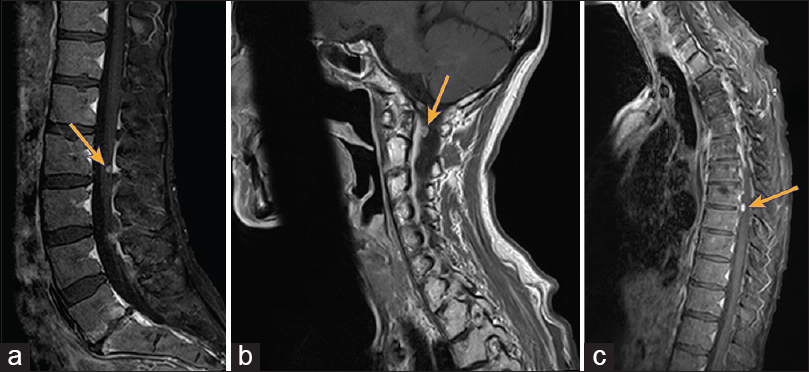
A 48-year-old woman presented with a large sinonasal mass with extensive intracranial extension through the cribriform plate into the anterior cranial fossa [
Spinal imaging revealed multiple dural-based enhancing lesions in the cervical, thoracic, and lumbar spine with possible osseous involvement, consistent with drop metastasis [
Figure 5
Photomicrographs from spinal mass at T9–10. H and E staining shows well-defined clusters of cells separated by a fibrovascular stroma (a). Higher magnification (b) shows small, round cells with large nuclei and scant cytoplasm. Immunohistochemical staining for synaptophysin, a neuronal cell marker, is strongly positive in all lesional cells (c) and is completely within the cytoplasm (d). Staining for CD45 (e), a lymphoid tissue marker, and cytokeratin, a marker for adenocarcinoma, were both negative. Magnification: (a and c) ×2, (b, d–f) ×40
The patient initially began a course of systemic carboplatin, vincristine, and lomustine, as well as intrathecal methotrexate. She ultimately completed the intrathecal methotrexate with carboplatin, etoposide, and cyclophosphamide. Follow-up imaging 16 months after the initiation of chemotherapy revealed resolution of the metastatic lesions and no radiographic appearance of residual disease, 9 years after her original diagnosis. She continues to receive surveillance for the recurrent disease by evaluation of her cerebrospinal fluid (CSF) every 3 months.
DISCUSSION
Even with aggressive therapy, esthesioneuroblastomas exhibit a propensity for both local and distant recurrence. They can disseminate via direct invasion into the brain parenchyma and metastasize through lymphatic, hematogenous, or leptomeningeal spread. Local recurrence and metastatic disease are seen in 50–60% and 10–62% of patients, respectively.[
The rarity of leptomeningeal dissemination in esthesioneuroblastoma has made it challenging to generate appropriate prospective studies to establish treatment protocols. Neuro-oncologists rely on case reports and retrospective studies to understand the natural course of the disease, as well as the response to treatments. Packer et al.[
This treatment protocol, which we used in our second patient, stemmed from a treatment designed for medulloblastoma displaying CNS metastasis.[
CONCLUSION
Although rare, distant metastasis from leptomeningeal dissemination in esthesioneuroblastoma can occur and portend a poor prognosis. We describe two patients with esthesioneuroblastoma treated previously with multimodal therapy who developed distal metastatic recurrence, one who went on to have long-term success. The possibility of leptomeningeal dissemination should be kept in mind when monitoring patients with esthesioneuroblastoma and early detection from surveillance CSF analysis should be the goal. Understanding the drivers of leptomeningeal dissemination in PNETs may hold the key to developing successful treatment strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Meghan Driscoll, M.D., for assisting with the acquisition of the pathology slides, Kristin Kraus, M.Sc., for her editorial assistance in the preparation of this paper, and Jennie Williams, M.A., for preparation of the figures.
References
1. Arnold PM, Habib A, Newell K, Anderson KK. Esthesioneuroblastoma metastatic to the thoracic intradural and extradural space. Spine J. 2009. 9: e1-5
2. Bailey BJ, Barton S. Olfactory neuroblastoma: Management and prognosis. Arch Otolaryngol Head Neck Surg. 1975. 101: 1-
3. Becker LE, Hinton D. Primitive neuroectodermal tumors of the central nervous system. Hum Pathol. 1983. 14: 538-50
4. Berger L, Luc R. Olfactory esthesioneuroepithelioma. Bull l’Assoc Franc Pour l’Etude Cancer. 1924. 13: 410-20
5. Bogucki J, Taraszewska A, Czernicki Z. Pure distant, leptomeningeal metastasis of esthesioneuro-epithelioma. Acta Neurochir (Wien). 2004. 146: 1043-5
6. Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: A general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1997. 17: 2683-706
7. Buohliqa L, Upadhyay S, Nicolai P, Cavalieri R, Dolci RL, Prevedello D. Possible esthesioneuroblastoma metastasis to paranasal sinuses: Clinical report and literature review. Head Neck. 2015. p.
8. Chamberlain MC, Dirr L. Involved-field radiotherapy and intra-Ommaya methotrexate/cytarabine in patients with AIDS-related lymphomatous meningitis. J Clin Oncol. 1993. 11: 1978-84
9. Chamberlain MC. Treatment of intracranial metastatic esthesioneuroblastoma. Cancer. 2002. 95: 243-8
10. Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: A meta-analysis and review. Lancet Oncol. 2001. 2: 683-90
11. Foote RL, Morita A, Ebersold MJ, Olsen KD, Lewis JE, Quast LM. Esthesioneuroblastoma: The role of adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 1993. 27: 835-42
12. Howell MC, Branstetter BF, Snyderman CH. Patterns of regional spread for esthesioneuroblastoma. AJNR Am J Neuroradiol. 2011. 32: 929-33
13. Hwang SK, Paek SH, Kim DG, Jeon YK, Chi JG, Jung HW. Olfactory neuroblastomas: Survival rate and prognostic factor. J Neurooncol. 2002. 59: 217-26
14. Jenkins NC, Kalra RR, Dubuc A, Sivakumar W, Pedone CA, Wu X. Genetic drivers of metastatic dissemination in sonic hedgehog medulloblastoma. Acta Neuropathol Commun. 2014. 2: 85-
15. Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976. 37: 1571-6
16. Klepin HD, McMullen KP, Lesser GJ. Esthesioneuroblastoma. Curr Treat Options Oncol. 2005. 6: 509-18
17. Mohindra S, Tripathi M, Mohindra S, Savardekar A, Radotra BD. Multiple intradural spinal metastases of esthesioneuroblastoma: A case report. Br J Neurosurg. 2015. 29: 579-81
18. Packer RJ, Sutton LN, Goldwein JW, Perilongo G, Bunin G, Ryan J. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg. 1991. 74: 433-40
19. Rodas RA, Erkman-Balis B, Cahill DW. Late intracranial metastasis from esthesioneuroblastoma: Case report and review of the literature. Neurosurgery. 1986. 19: 622-7
20. Shaari CM, Catalano PJ, Sen C, Post K. Central nervous system metastases from esthesioneuroblastoma. Otolaryngol Head Neck Surg. 1996. 114: 808-12
21. Shirzadi AS, Drazin DG, Strickland AS, Bannykh SI, Johnson JP. Vertebral column metastases from an esthesioneuroblastoma: Chemotherapy, radiation, and resection for recurrence with 15-year followup. Case Rep Surg 2013. 2013. p. 107315-
22. Theilgaard SA, Buchwald C, Ingeholm P, Kornum Larsen S, Eriksen JG, Sand Hansen H. Esthesioneuroblastoma: A Danish demographic study of 40 patients registered between 1978 and 2000. Acta Otolaryngol. 2003. 123: 433-9
23. Yu T, Xu YK, Li L, Jia FG, Duan G, Wu YK. Esthesioneuroblastoma methods of intracranial extension: CT and MR imaging findings. Neuroradiology. 2009. 51: 841-50