- Department of Neurosurgery, National Center for Child Health and Development, Tokyo, Japan.
Correspondence Address:
Shotaro Ogawa, Department of Neurosurgery, National Center for Child Health and Development, Tokyo, Japan.
DOI:10.25259/SNI_623_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shotaro Ogawa, Hideki Ogiwara. Cranial distraction osteogenesis for craniosynostosis associated with osteopetrosis: A case report. 13-Oct-2023;14:368
How to cite this URL: Shotaro Ogawa, Hideki Ogiwara. Cranial distraction osteogenesis for craniosynostosis associated with osteopetrosis: A case report. 13-Oct-2023;14:368. Available from: https://surgicalneurologyint.com/surgicalint-articles/12589/
Abstract
Background: Osteopetrosis is a rare disease characterized by systemic osteosclerosis and hematopoietic disturbances. Childhood-onset cases are often accompanied by hydrocephalus and craniosynostosis; however, there have been no established treatments. We performed cranial distraction in a child with osteopetrosis who presented with craniosynostosis and intracranial hypertension.
Case Description: The patient was a 4-year-1-month-old boy. His pregnancy and birth were normal, but at 4 months of age, he was diagnosed with osteopetrosis based on generalized osteosclerosis and family history. A computed tomography scan of the head revealed early sagittal suture fusion and ventricular enlargement. A ventriculoperitoneal shunt was placed for intracranial hypertension; however, slit ventricle syndrome ensued and pansynostosis developed. To improve uncontrolled high intracranial pressure, cranial distraction was performed for intracranial volume expansion. No perioperative hemorrhagic or infectious complications were observed. After the start of distraction, the intracranial pressure gradually decreased, and clinical findings such as disturbance of consciousness and bradycardia disappeared. Bone regeneration in the defect site was good, and the extension device was removed 6 months after the operation.
Conclusion: For osteopetrosis with poorly controlled intracranial hypertension, cranial distraction was considered to be an effective treatment.
Keywords: Cranial distraction osteogenesis, Craniosynostosis, Intracranial hypertension, Osteopetrosis
INTRODUCTION
Osteopetrosis is a rare disease characterized by systemic osteosclerosis, hematopoietic dysfunction, and fragility. Based on clinical findings, it is classified into early-onset and late-onset forms. The early-onset type develops at birth or in infancy and causes hardening and deformation of the skull, impaired vision and hearing, and impaired mental development. The early-onset type entails a poor prognosis. The late-onset type is found incidentally on X-rays, and central nervous system disorders are rare. In addition, an intermediate type and a disease type accompanied by renal tubular acidosis are reported.[
As for the genetic abnormality, more than half of the children with autosomal recessive infantile osteopetrosis have variants in the TCIRG1 gene.[
CASE DESCRIPTION
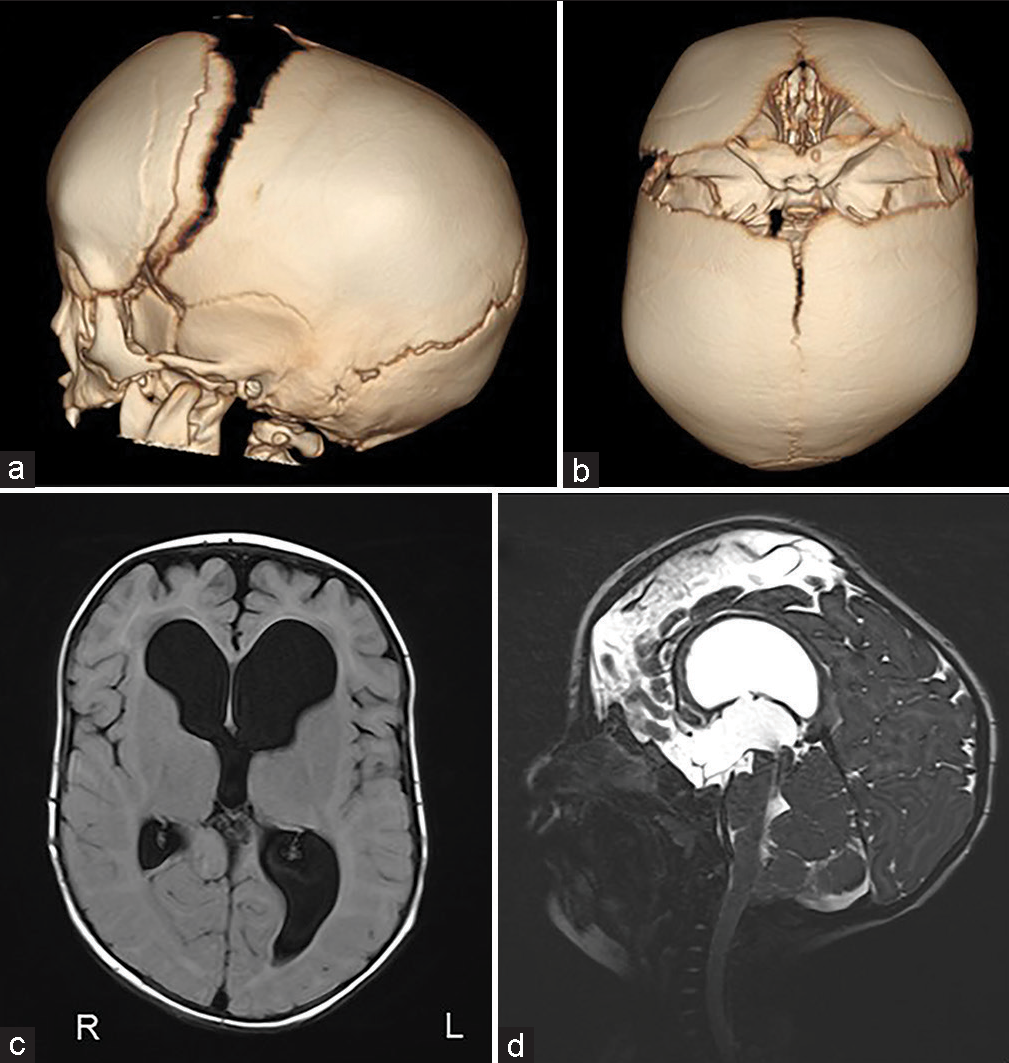
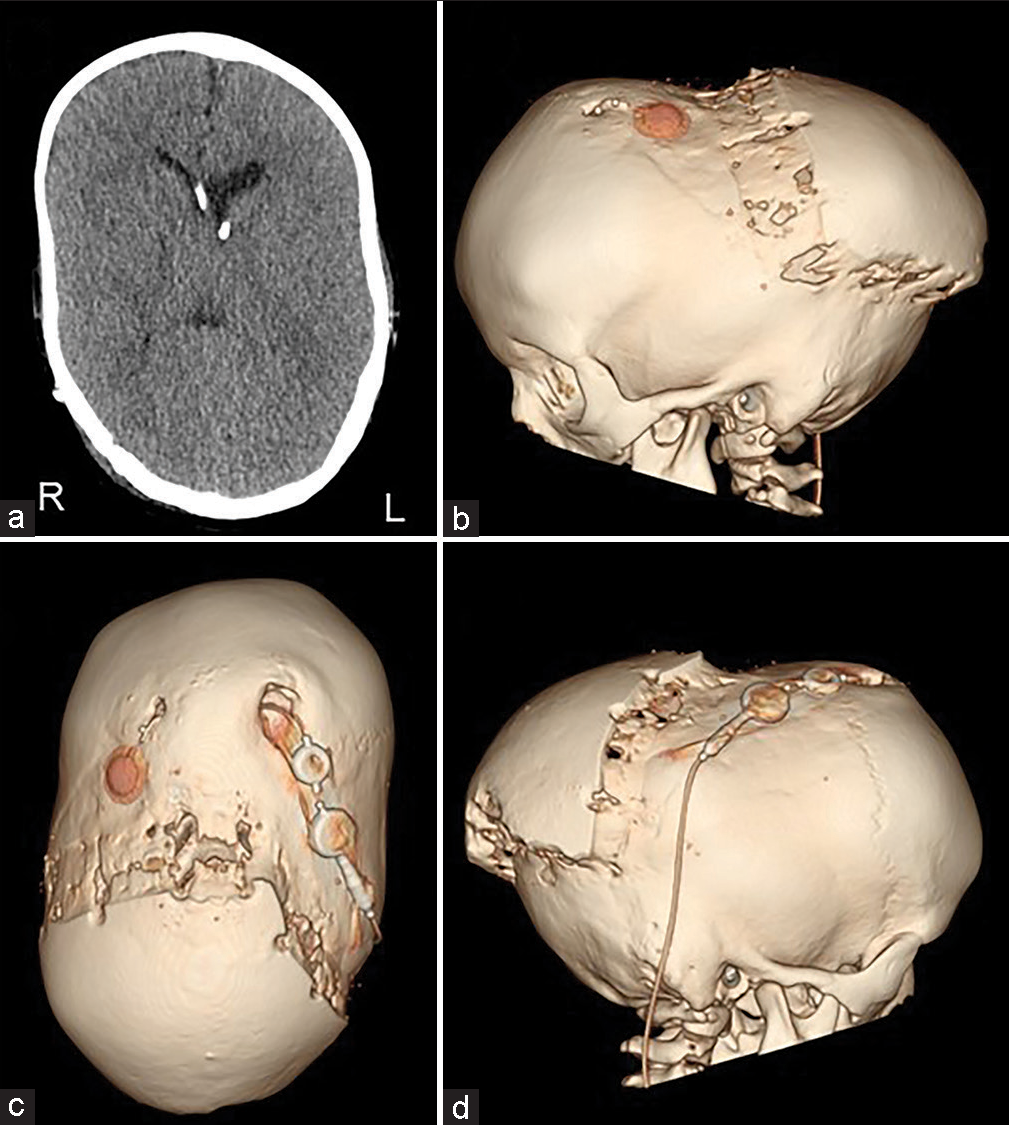
The patient was a 4-year-1-month-old boy. He was born at 40 weeks 0 days gestation weighing 3262 g. When he was 4 months old, severe atrophy of bilateral optic nerves was found due to his inability to pursue. The X-ray showed sclerosis of the whole-body bone. He was diagnosed with osteopetrosis because of a family history of his maternal grandmother’s brother dying of osteopetrosis at the age of five. The computed tomography (CT) scan showed early sagittal suture synostosis and ventricular enlargement [
The surgical procedure was performed through the coronal skin incision. In this case, because there was a possibility of bleeding that was difficult to control during the surgery, we started a transfusion of red blood cells and fresh frozen plasma at the beginning of the surgery in preparation for intraoperative bleeding.
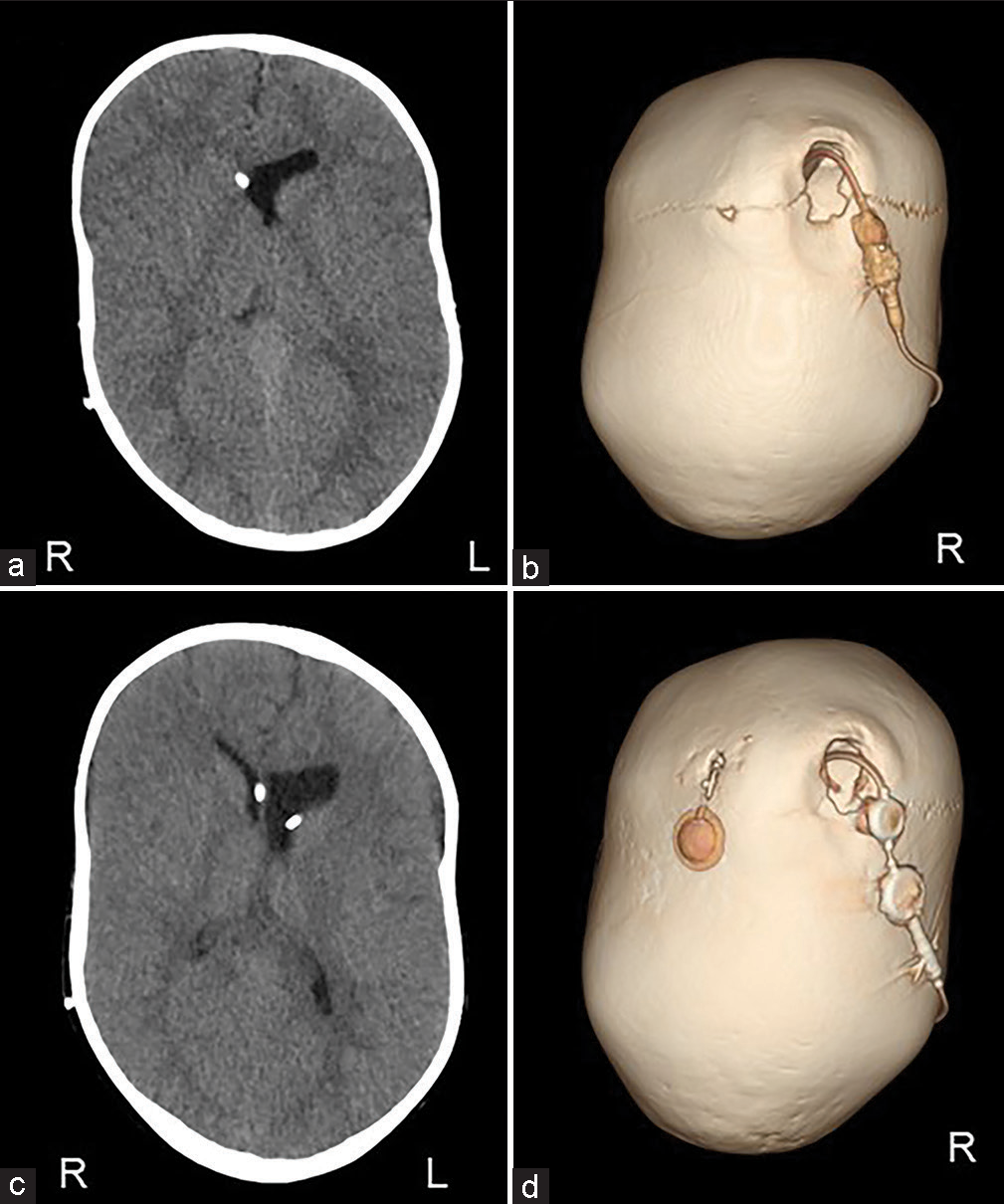
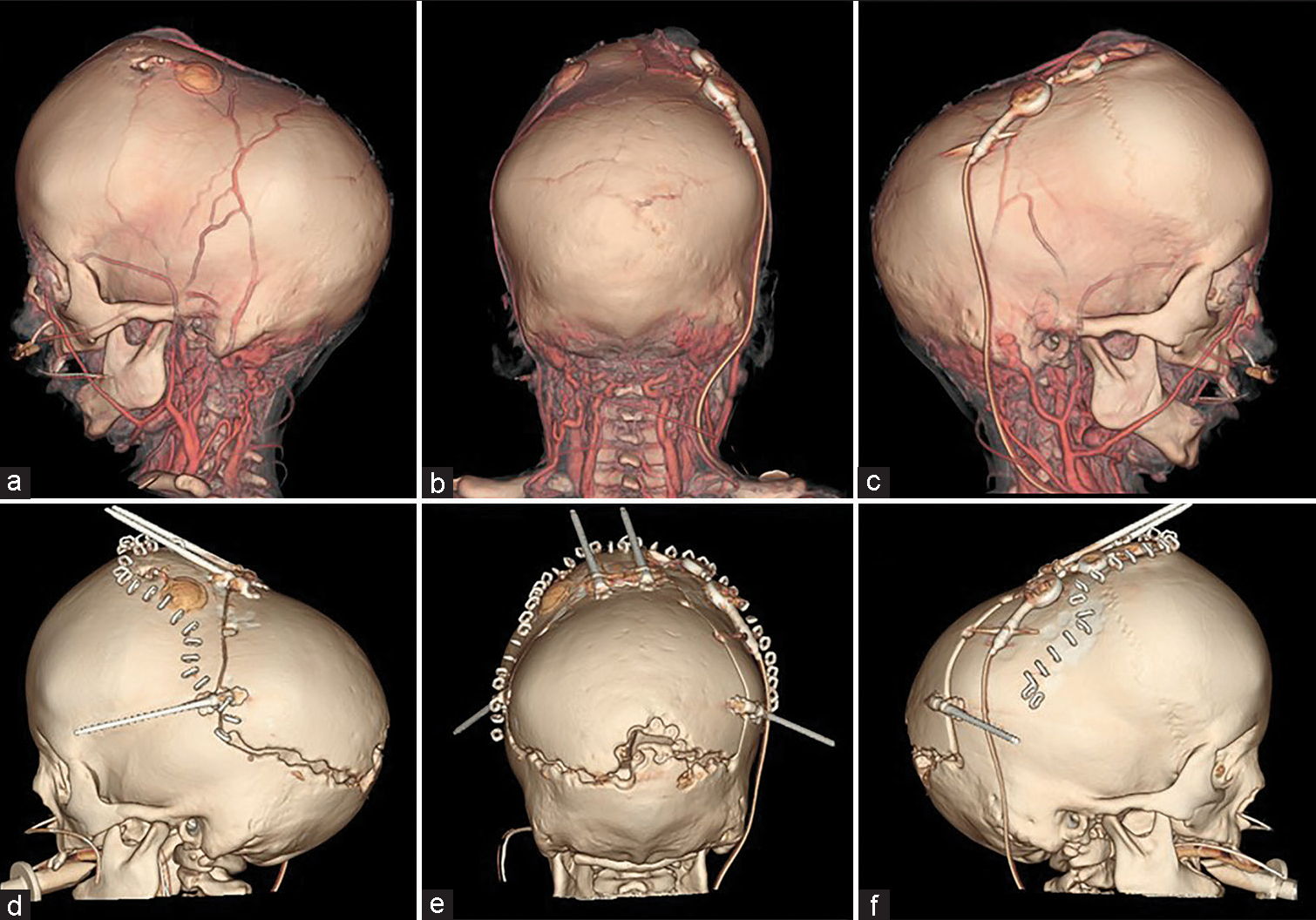
The scalp was reflected and the calvarial surface was exposed. A bilateral circumferential parieto-occipital osteotomy was performed. The osteotomy line was decided using neuronavigation. The cortical bone was very hard and thick, while the cancellous bone was relatively thin. The dura was fragile and strongly adhered to the bone. To prevent sinus injury, the osteotomy above and close to the superior sagittal sinus was performed gently using Kerrison rongeur. The osteotomy line was kept rostral to the transverse sinus. Four cranial distractors were fixed to the cranial bone. The sites of the distractors were decided so as not to interfere with the existing shunt and reservoir [
Figure 3:
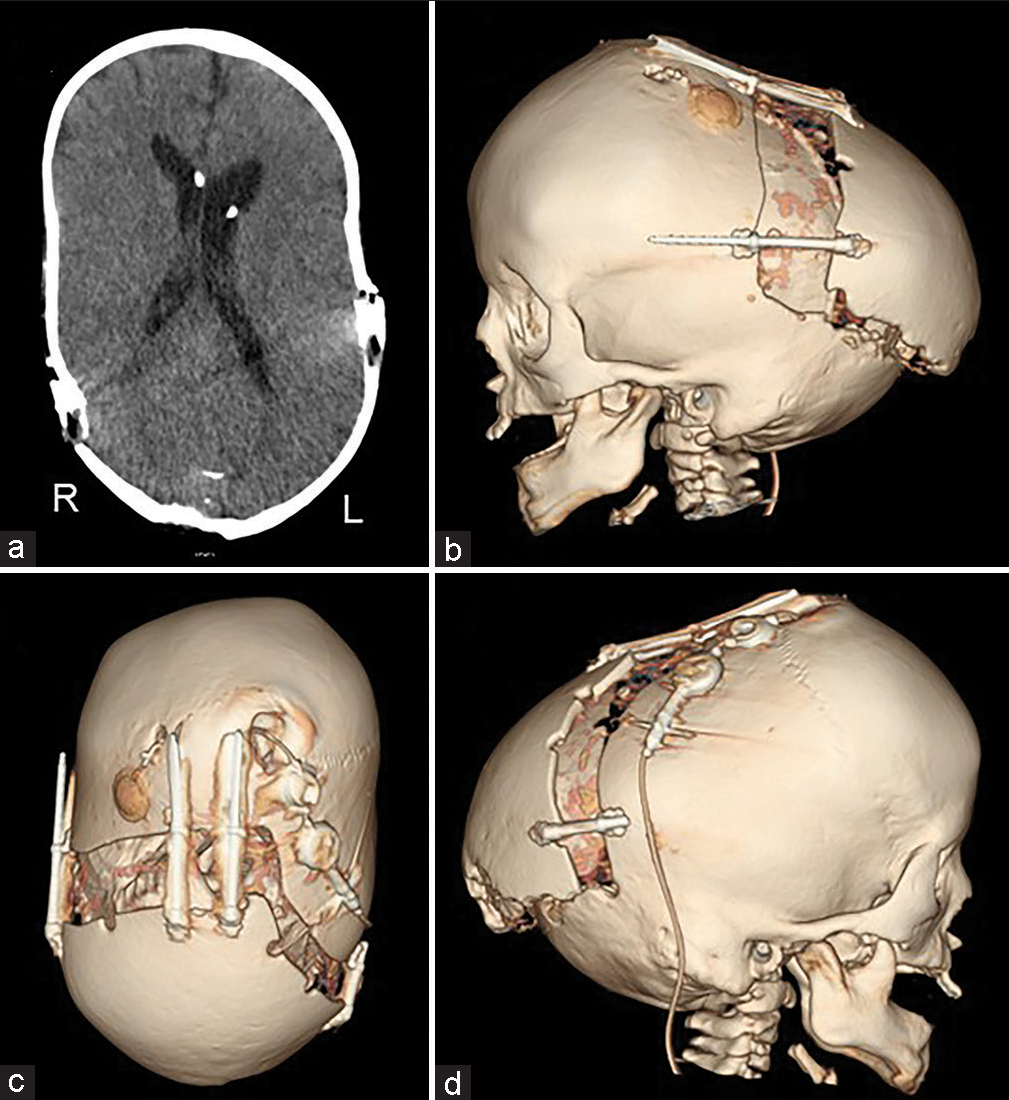
(a-c) Preoperative and (d-f) postoperative computed tomography. The osteotomy line was designed so as not to interfere with existing shunts and reservoirs. Furthermore, the emissary vein was preserved as much as possible. The osteotomy line was kept rostral to the transverse sinus because the adhesion between the bone and the dura mater was particularly strong near the sinus confluence.
No perioperative hemorrhagic complications or infections were observed. Cranial distraction was started on the 6th postoperative day and extended to a total of 25 mm (1 mm/day). The extension of the lateral distractor on the right side was terminated at 9 mm not to interfere with the shunt [
DISCUSSION
In this report, we performed cranial distraction osteogenesis for a child with osteopetrosis who presented with uncontrolled intracranial hypertension and obtained a favorable outcome.
Surgical treatment for osteopetrosis
In osteopetrosis, the dysfunction of osteoclasts leads to high bone density but susceptibility to fracture.[
Cranial expansion for osteopetrosis
In craniosynostosis associated with osteopetrosis, multiple factors are thought to contribute to intracranial hypertension. The increased thickness of the calvarium and the decrease in the cranial capacity are the causes of increased intracranial pressure.[
Necessity of gradual cranial distraction
Jamjoom et al. reported the case of a 4-year-old boy with osteopetrosis who had a cardiac arrest during one-stage cranial enlargement. They considered hypotension associated with sudden intracranial pressure drop, complication of Chiari malformation, and intraoperative bleeding as causes of cardiac arrest.[
Preservation of emissary veins
In osteopetrosis, venous outflow stenosis is considered to be one of the causes of hydrocephalus and increased intracranial pressure.[
Determination of craniotomy range and surgical technique
In cranial distraction surgery, a large bone flap that includes the caudal side of the torcula is often created to simultaneously decompress the venous sinuses.[
Meticulous detachment using Kerrison rongeur was effective to preserve the dura during the osteotomy and no bleeding from the sinus and postoperative CSF leakage occurred. Considering the fragility of the dura mater, it was considered necessary to delay the start timing of distraction. We delayed the start of distraction to the 6th postoperative day and no CSF leakage due to dural injury was observed throughout the course of distraction.
Treatment timing
It is difficult to judge when to perform cranial expansion surgery in the case of osteopetrosis-associated craniosynostosis and hydrocephalus. We usually perform a posterior vault expansion for craniosynostosis before considering drainage techniques such as VP shunt surgery. However, in patients with osteopetrosis, we should decide on the surgical method considering that they have a high risk of bleeding in surgery with bone manipulations. In this case, we performed a VP shunt before cranial expansion against intracranial hypertension that was poorly controlled by ETV and regular puncture drainage. We suggested that considering the risk of cranial distraction surgery, the VP shunt surgery and/or ETV was performed first, and the cranial distraction surgery may better be delayed until high intracranial pressure becomes uncontrollable even with the VP shunt.
CONCLUSION
We successfully performed cranial distraction osteogenesis for the patient with osteopetrosis associated with hydrocephalus and craniosynostosis. Cranial distraction osteogenesis is considered an effective treatment for uncontrolled intracranial hypertension with osteopetrosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akutsu N, Koyama J, Kawamura A, Nagashima T, Taniguchi M, Kohmura E. Endoscopic third ventriculostomy for hydrocephalus in osteopetrosis: A case report and review of the literature. Childs Nerv Syst. 2018. 34: 991-4
2. Baird PA, Robinson GC, Hardwick DF, Sovereign AE. Congenital osteopetrosis: An unusual cause of hydrocephalus. Can Med Assoc J. 1968. 98: 362-5
3. Chalhoub N, Benachenhou N, Rajapurohitam V, Pata M, Ferron M, Frattini A. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med. 2003. 9: 399-406
4. Cinalli G, Russo C, Vitulli F, Parlato RS, Spennato P, Imperato A. Changes in venous drainage after posterior cranial vault distraction and foramen magnum decompression in syndromic craniosynostosis. J Neurosurg Pediatr. 2022. 30: 1-12
5. Cleiren E, Bénichou O, Van Hul E, Gram J, Bollerslev J, Singer FR. Albers-Schönberg disease (autosomal dominant osteopetrosis, Type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet. 2001. 10: 2861-7
6. Dhamija B, Pettorini BL, Solanki G. Endoscopic third ventriculostomy for the treatment of osteopetrosis-related hydrocephalus: A case-based update. Childs Nerv Syst. 2011. 27: 1861-5
7. Dowlati D, Winston KR, Ketch LL, Quinones R, Giller R, Frattini A. Expansion cranioplasty with jackscrew distracters for craniosynostosis and intracranial hypertension in transplanted osteopetrosis. Pediatr Neurosurg. 2007. 43: 102-6
8. Even-Or E, Stepensky P. How we approach malignant infantile osteopetrosis. Pediatr Blood Cancer. 2021. 68: e28841
9. Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000. 25: 343-6
10. Gerritsen EJ, Vossen JM, van Loo IH, Hermans J, Helfrich MH, Griscelli C. Autosomal recessive osteopetrosis: Variability of findings at diagnosis and during the natural course. Pediatrics. 1994. 93: 247-53
11. Ghali GZ, Zaki Ghali MG, Ghali EZ, Srinivasan VM, Wagner KM, Rothermel A. Intracranial venous hypertension in craniosynostosis: Mechanistic underpinnings and therapeutic implications. World Neurosurg. 2019. 127: 549-58
12. Grossman R, Feldman Z. Bone growth causing ventriculoperitoneal shunt malfunction in a patient with osteopetrosis. Case report. J Neurosurg. 2004. 100: 530-1
13. Jamjoom AA, Jamjoom BA, Waliuddin AR, Jamjoom AB. Lessons from a case of osteopetrosis oxycephaly and Chiari Type I malformation: A case report. Cases J. 2009. 2: 6787
14. Kishore S, Khaja MS, Thornburg B, Sharma AM, Knuttinen MG, Shamoun F. Antithrombotic therapy after venous interventions: AJR expert panel narrative review. AJR Am J Roentgenol. 2022. 219: 175-87
15. Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T. Mutations in the a3 subunit of the vacuolar H+-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet. 2000. 9: 2059-63
16. Krimmel M, Niemann G, Will B, Reinert S. Surgical correction of craniosynostosis in malignant osteopetrosis. J Craniofac Surg. 2004. 15: 218-20
17. Lee A, Cortez S, Yang P, Aum D, Singh P, Gooch C. Neonatal hydrocephalus: An atypical presentation of malignant infantile osteopetrosis. Childs Nerv Syst. 2021. 37: 3695-703
18. Lundar T, Bakke SJ, Nornes H. Hydrocephalus in an achondroplastic child treated by venous decompression at the jugular foramen. Case report. J Neurosurg. 1990. 73: 138-40
19. Piret SE, Gorvin CM, Trinh A, Taylor J, Lise S, Taylor JC. Autosomal dominant osteopetrosis associated with renal tubular acidosis is due to a CLCN7 mutation. Am J Med Genet A. 2016. 170: 2988-92
20. Ramírez A, Faupel J, Goebel I, Stiller A, Beyer S, Stöckle C. Identification of a novel mutation in the coding region of the grey-lethal gene OSTM1 in human malignant infantile osteopetrosis. Hum Mutat. 2004. 23: 471-6
21. Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009. 4: 5
22. Stella I, Vinchon M, Guerreschi P, De Berranger E, Bouacha I. Case update on cranial osteopetrosis: Which is the role of the neurosurgeon?. Childs Nerv Syst. 2017. 33: 2181-6
23. Wilson CJ, Vellodi A. Autosomal recessive osteopetrosis: Diagnosis, management, and outcome. Arch Dis Child. 2000. 83: 449-52
24. Wu CC, Econs MJ, DiMeglio LA, Insogna KL, Levine MA, Orchard PJ. Diagnosis and management of osteopetrosis: Consensus guidelines from the osteopetrosis working group. J Clin Endocrinol Metab. 2017. 102: 3111-23