- Department of Neurosurgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States
- Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States
- Department of Neurosurgery, University of Baghdad, Baghdad, Iraq
- Department of Neurosurgery, College of Medicine, University of Baghdad, Baghdad, Iraq
- Department of Neurosurgery, University of Mustansiriyah, Baghdad, Iraq
- Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq
- Department of Neurology, University of Pittsburgh Medical Center Stroke Institute, Pittsburgh, Pennsylvania, United States
Correspondence Address:
Samer S. Hoz, Department of Neurosurgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States.
DOI:10.25259/SNI_531_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samer S. Hoz1, Li Ma2, Ahmed Muthana3, Mahmood Falah Al-Zaidy4, Fatimah Oday Ahmed5, Mustafa Ismail6, Rachel C. Jacobs1, Prateek Agarwal1, Alhamza R. Al-Bayati7, Raul G. Nogueira7, Michael J. Lang1, Bradley A. Gross1. Cranial nerve palsies and intracranial aneurysms: A narrative review of patterns and outcomes. 09-Aug-2024;15:277
How to cite this URL: Samer S. Hoz1, Li Ma2, Ahmed Muthana3, Mahmood Falah Al-Zaidy4, Fatimah Oday Ahmed5, Mustafa Ismail6, Rachel C. Jacobs1, Prateek Agarwal1, Alhamza R. Al-Bayati7, Raul G. Nogueira7, Michael J. Lang1, Bradley A. Gross1. Cranial nerve palsies and intracranial aneurysms: A narrative review of patterns and outcomes. 09-Aug-2024;15:277. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13033
Abstract
Background: Cranial nerve palsy (CNP) in patients with intracranial aneurysms (IAs) can impose significant burdens on a patient’s quality of life. The literature has a paucity of reviews addressing patterns of overall reported cranial nerve (CN) involvement and outcomes in patients with IA.
Methods: The literature systematically reviewed CNP at presentation in the setting of IA using PubMed, Web-of-Science, and Scopus according to the PRISMA guidelines.
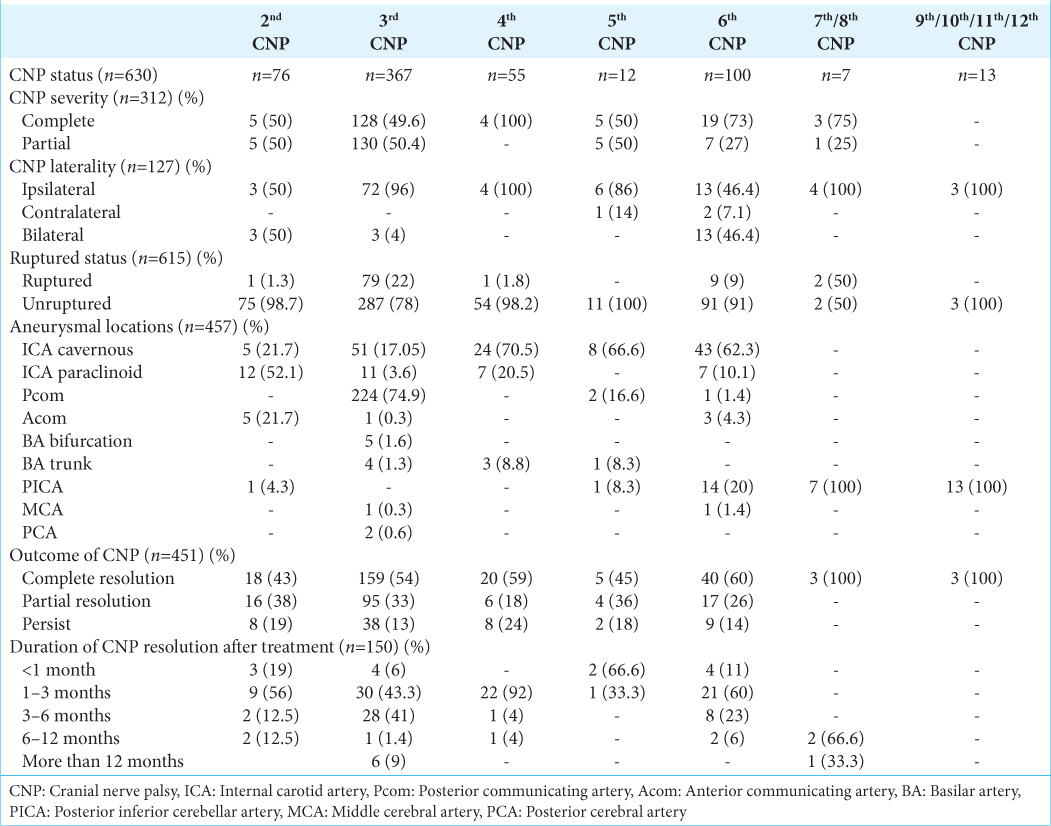
Results: Fifty-two studies reported a total of 513 patients with IA and 630 CNPs observed at presentation: oculomotor (58.25%), abducent (15.87%), optic (12.06%), trochlear (8.7%), and trigeminal (1.9%). Most common aneurysms are located in a posterior communicating artery (46%) and cavernous internal carotid artery (29.2%). Trends of CNP based on the rupture status of IAs showed that 80% were associated with unruptured IAs and 20% with ruptured IAs. Post-treatment of IA, 55% of patients had complete resolution of CNP, with most (89%; n = 134) resolving within the first 6 months. Stratified by CNP type: Complete resolution rate is 100% in CN VII–IX, 60% in CN VI, 59% in CN IV, 54% in CN III, 45% in CN V, and 43% in CN II.
Conclusion: In patients with cranial nerve palsies attributed to IAs, the location and rupture status of the aneurysm could determine the type and severity of the nerve palsy. Most patients experienced favorable outcomes in terms of their resolution and long-term function of the CNP after treatment of the IA.
Keywords: Aneurysm-induced cranial nerve palsy, Cranial nerve palsy, Cranial neuropathy, Intracranial aneurysm, Oculomotor palsy, Posterior communicating artery
INTRODUCTION
Cranial nerve palsy (CNP) in patients with intracranial aneurysms (IAs) can be part of the natural history of the pathology or a result of its treatment. As a presenting finding, CNP can be a component of the constellation of symptoms encountered in patients with cerebral aneurysms.[
Cranial nerve dysfunction can be attributed to either injury to the nerve itself along its course, blood supply, or origin and nuclei within the brainstem.[
IAs can present with CNP (or palsies) that impose a significant burden on a patient’s quality of life. The literature showed a paucity of reviews detailing the patterns of CNP and related aneurysmal characteristics for each cranial nerve (CN) in the setting of IAs. We systematically reviewed the literature on CNP at their presentation in the setting of IAs. We comprehensively summarized the literature on CNP associated with IAs, aiming to delineate the patterns and recovery outcomes of CNP in patients with IAs. We are trying to answer the following four main questions: (1) What are the potential IA locations that can serve as culprits to that CN? (2) What are the patterns of CNP for each location of IAs, for each CNP (3) What are the trends of CNP based on the rupture status of IAs? (4) What is the outcome and duration of palsy for each CN?
MATERIALS AND METHODS
Literature search
A systematic review was executed in adherence to the PRISMA extension for scoping review protocols.[
Study selection
Inclusion and exclusion benchmarks were predetermined. Articles were selected if they encompassed at least one patient manifesting CNP and diagnosed with an intracranial aneurysm that received treatment. CNP was defined as a clinical diagnosis denoting the dysfunction of any cranial nerve, as recognized or reported by the respective authors. For intracranial aneurysms, the diagnosis was based on computed tomography angiography, magnetic resonance angiography, or digital subtraction angiography. Duplicated studies were excluded. The presence of single or multiple CNPs was deemed acceptable. However, traumatic CNPs were excluded from the study. Extracranial aneurysms, untreated aneurysms, and multiple aneurysms were excluded from the study. Exclusions were also for studies with no relevant data on CNP or aneurysmal characteristics, non-English language-based studies, conference abstracts, technical reports, book chapters, letters to the editor, editorials, radiological studies, anatomical studies, or those employing animal or cadaveric subjects. Although review articles were excluded from the review, references to these studies were screened to determine whether other original studies were relevant. Based on the rarity of the association of CNP at presentation and IAs, we decided to include case series and reports of less than ten patients. The purpose is to provide an inclusive review that aids in answering the critical questions we aimed for.
Four distinct reviewers (A.M., F.O., M.A., and M.I.) systematically scrutinized the titles and abstracts of the retrieved articles, advancing to comprehensive text analysis for those aligning with the stipulated inclusion criteria. Discrepancies, when encountered, were mediated by a fifth reviewer (S.H.). Following the pre-established guidelines, relevant articles were integrated, and their reference lists were examined for additional pertinent studies.
Data extraction
Reviewers A.M., F.O., M.A., and M.I. initiated the data extraction, which was subsequently cross-checked for accuracy by reviewer S.H. Extracted parameters included Author - Year, Country of Origin, Study Configuration, Population Size, Age Demographics, Gender Distribution, Status of Aneurysm Rupture, Aneurysm characteristics, CNP characteristics, Intervention Methodology, Glasgow Coma scale, CNP outcome, Comprehensive CNP Outcome Overview, Monitoring Span (in months) for CN Functionality, and Duration Until CNP Resolution. In the current investigation, evidence classification and weighting adhered to the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence delineated by Howick.[
Data synthesis, quality assessment, and statistical analysis
For the variables under consideration, analyses of proportions were undertaken to secure aggregated estimates. Two separate authors (S.H. and M.I.) meticulously assessed the risk of bias inherent in the included studies through the JBI checklists. Each article’s evidence caliber was ascertained in alignment with the 2011 OCEBM criteria.[
RESULTS
Study selection
We incorporated 52 studies that reported a total of 513 patients with intracranial aneurysms and CNPs observed at presentation. Of those 52 studies, 15 were clinical cohorts with more than ten patients, which revealed 408 patients (80%). The other articles include small case series or reports that denote 105 cases (20%).
CNP characteristics
The incidence of CNP in IAs ranges from 0.1 to 7%, based on a few studies that include the total number of aneurysms cohorts.[
Aneurysm location characteristics
Based on aneurysmal location, the characteristics of 513 intracranial aneurysms in patients with CNP at presentation are detailed in
In an evaluation of Pcom aneurysms, a cohort of 236 subjects was considered. The majority were unruptured (72.4%, n = 150), while 27.5% (n = 57) were ruptured. The majority exhibited CNP associated with the third nerve palsy (98.6%; n = 224), and scattered cases were observed with the fifth (0.8%) and sixth CNP (0.4%). For cavernous ICA aneurysms, 150 subjects were studied. All were unruptured (100%). The third nerve palsy was the most frequent (39%; n = 51), followed by the sixth nerve palsy in 33% (n = 43) and the fourth nerve palsy in 18% (n = 24). Furthermore, 69 patients with paraclinoid ICA aneurysms were studied, all of which were unruptured (100%). CNP analysis revealed that the second nerve was the most commonly affected in 32.4% (n = 12) of cases, followed by the third nerve in 30% (n = 11) of cases, and the fourth and sixth nerves affected in 20% (n = 7) for each nerve palsy.
PICA aneurysms were seen in 32 patients who had CNP at presentation. With 16.6% (n = 3) being unruptured and 83.3% (n = 15) ruptured. The sixth nerve was affected at a rate of 39% (n = 14), followed by the tenth nerve at 27.7% (n = 10). In addition, PICA aneurysms were the single highest cause of lower CNP in our study (55.5%, n = 20).
For BA trunk aneurysm-associated CNPs, eight cases were identified, and all of them were unruptured (100%). Nearly 50% of these cases were with third nerve palsy, 37.5% with fourth palsy, and 12.5% with fifth nerve impairment.
Aneurysm rupture status characteristics
Characteristics of patients with CNP in relation to aneurysm rupture status are presented in
Any study in our review cannot confirm the exact mechanism of CNP. However, some studies suggest a peculiar description of their cohorts. Direct compression was identified as the predominant mechanism causing CNP in the unruptured group, accounting for 98% of the cases. In contrast, indirect cranial nerve injury due to hemorrhage was the most commonly reported mechanism observed in 63% of ruptured group cases, with direct compression credited as the cause in the remainder.
DISCUSSION
The cranial nerves can be affected as a part of the pathogenesis of intracranial aneurysms. The intracranial course and the proximity to surrounding vascular structures determine the degree of palsy in the aneurysm location. Along with aneurysmal location, the rupture status can play a pivotal role in the causation of CNP.[
The potential aneurysm sites that serve as culprits for each CNP
Based on all the recruited cases, the IA locations that can cause 3rd CNP at presentation include mainly Pcom and cavernous ICA (91%), followed by paraclinoid, Acom, PICA, BA, posterior cerebral artery, Acom, and middle cerebral artery (MCA) aneurysms in order of frequency. For the 6th CNP, cavernous ICA and PICA aneurysms represent 82% of the culprit locations, followed by other rarer sites such as paraclinoid, Acom, Pcom, and MCA aneurysms. The cavernous and paraclinoid IAs were the most common cause for both the 2nd and 4th CNP, while cavernous and Pcom IAs were the most common culprits for the 5th CNP. Finally, PICA aneurysms were the only aneurysm location reported to have lower cranial nerve palsies at presentation [
The cranial nerves that are mostly linked to intracranial aneurysms through the literature are the third CNP. The majority of oculomotor palsies are attributed to intracranial aneurysms related to the anterior circulation arteries.[
When we analyzed the recovery of 3rd nerve palsy, it was complete in 54%, partial in 33% of cases, while 13% were with persistent palsy. We hypothesize that the diversity in recovery outcomes may stem from the distinct mechanisms by which aneurysms induce third nerve palsy, whether they are ruptured or unruptured, and how long this nerve was involved, as previously discussed.
Anatomically, the abducent nerve follows a peculiar intracranial pathway and is susceptible to being affected by an enlarged aneurysm along the distance from the brainstem to the orbit. Among all cases of abducent nerve lesions, some studies suggest that 3.6% of 6th nerve palsies can be attributed to intracranial aneurysms.[
Figure 2:
Artistic depiction showing examples of intracranial aneurysms related cranial nerve palsy. BA: Basilar artery, AICA: Anterior inferior cerebellar artery, PICA: Posterior inferior cerebellar artery, SCA: Superior cerebellar artery, PCA: Posterior cerebral artery, III: Oculomotor Nerve, IV: Trochlear Nerve, V: Trigeminal Nerve, VI: Abducens Nerve, VII: Facial Nerve, VIII: Vestibulocochlear Nerve, IX: Glossopharyngeal Nerve, X: Vagus Nerve. Illustration prepared by Ahmed Muthana and courtesy of Samer Hoz.
The lower cranial nerve palsies (7th–12th) are not frequently related to intracranial aneurysms at presentation. Out of all CNPs analyzed in our study, lower cranial nerves were affected in only 13 patients. Almost all these nerve dysfunctions are attributed to posterior circulation aneurysms, particularly PICA aneurysms. Due to the proximity to the brainstem and the origin of lower cranial nerves, impairment of these nerves may occur at presentation time of posterior circulation aneurysms.[
The patterns of CNP for each location of intracranial aneurysms
Aneurysmal location was most frequently Pcom (46%), followed by cavernous ICA (29.2%), Paraclinoid (13.4%), PICA (6.2%), and BA (1.5%) IAs. For Pcom aneurysms, the reported CNP includes 3rd CN (98.6%), in addition to rare cases involving 5th and 6th CNs. Regarding cavernous ICA aneurysms, the analyzed CNP cases include 3rd CN (39%), 6th (33%), and 4th (18%), in addition to rare reports for 5th and 2nd CNs. For paraclinoid aneurysms, the reported CNP includes 2nd (33%), 3rd (39%), 4th (18%), and 6th (18%) cranial nerves. Those with PICA aneurysms can result in CNP for most lower cranial nerves in addition to the 6th CNP well. In addition, the reported CNP for BA aneurysms includes the 3rd, 4th, and 5th CNP [
The precise location of an intracranial aneurysm, whether related to the anterior or posterior circulation, has a pivotal role in the determination of which cranial nerves could potentially be affected. The relationship between the specific aneurysmal locations and cranial nerves is intricately tied to the anatomical proximity of these aneurysms to the cranial nerves. Furthermore, the proximity of the aneurysm to the brainstem and its relationship with the cavernous sinus also carry significance in this clinical context.[
For anterior circulation aneurysms, 25% of intracranial aneurysms arise from ICA at the Pcom origin, making this site the second most frequent location after Acom aneurysms.[
Concerning posterior circulation aneurysms they encompass approximately 10–15% of the entire spectrum of cerebral aneurysms.[
The trends of CNP based on the rupture status of IAs
When comparing the CNP trends in the ruptured against unruptured groups of IAs, the affected cranial nerve was found to be consistent in both groups as the 3rd CN followed by the 6th CN. In addition, the 2nd and 4th CN represent approximately a quarter of CNP in the unruptured group. Moreover, the complete resolution of CNP is more common after ruptured IAs as compared to unruptured IAs (62% vs. 50%, respectively). Finally, the unruptured IAs have an earlier resolution time for their CNP when compared to ruptured aneurysms [
The underlying mechanism of CNP resulting from unruptured aneurysms differs from those seen in cases of ruptured aneurysms, with different potential theories proposed explaining the pathogenesis of this correlation through the literature. In ruptured aneurysms, damage to cranial nerves can be attributed to direct trauma from the jet of blood, localized hematoma formation, and/or irritation caused by the presence of blood in subarachnoid space.[
The outcome and duration of palsy for each cranial nerve in the setting of IAs
The highest rate of complete functional recovery from CNP was observed in cases involving the sixth cranial nerve, with a recovery rate of 60% (n = 40). This was followed by the fourth cranial nerve with a recovery rate of 59% (n = 20), the third cranial nerve at 54% (n = 159), the fifth nerve at 45% (n = 5), and the second cranial nerve at 43% (n = 18). The resolution duration was <6 months in most cases (89.3%; n = 134) [
In summary, the most affected cranial nerve was the third nerve, representing 58.25% of the total CNP case at presentation in the setting of IAs, followed by the 6th, 2nd, 4th, 5th, and lower cranial nerves in decreasing frequency. The IA locations that can cause 3rd CNP at presentation include mainly Pcom and cavernous ICA. For the 6th CNP, cavernous and PICA aneurysms represent the most common culprit locations. The ICA cavernous and ICA paraclinoid IAs were the most common cause for both the 2nd and 4th CNP. The patterns of CNP associated with Pcom aneurysms include 3rd CN, most frequently followed by 5th and 6th CNs. Regarding cavernous ICA aneurysms, the pattern of CNP cases includes 3rd, 6th, and 4th in order of frequency. Those with PICA aneurysms can result in CNP for most lower cranial nerves in addition to the 6th CNP well. The trends of CNP based on the rupture status of IAs showed that the most affected cranial nerve in both ruptured and unruptured groups is the 3rd CN, followed by the 6th CN. In addition, the complete resolution of CNP is more common after ruptured IAs as compared to unruptured IA. Finally, the unruptured IAs have an earlier resolution time for their CNP when compared to ruptured aneurysms. The highest rate of complete functional recovery from CNP was observed in cases involving the sixth cranial nerve, followed by the fourth cranial nerve. The resolution duration was <6 months in most cases of CNP.
Limitations
Our study has certain limitations. For aneurysms, particularly ruptured ones, factors such as distant SAH vasospasm in cranial nerve-supplying arteries and adhesions remain influential, even in carefully selected cases based on anatomical correlations and timing. Our focus on English-language articles may have excluded relevant research in other languages, possibly introducing language bias. Most reviewed articles were retrospective, introducing inherent selection bias due to preexisting data. Although we found no evidence of publication bias, institutional reporting bias cannot be entirely ruled out. Another limitation is that the majority of included studies were cohort analyses of more than ten cases, which contributed to 80% of the cases in our review. However, 20% represent case series and reports of less than ten patients. This is a limitation to review from generalizability, incidence, and prevalence perspectives. Arguably, this study aims to maximize the inclusivity of the reported cases to provide a thorough descriptive outcome that can be valuable on the other arm of the equation. Despite these limitations, our review offers valuable insights into the CNPIA relationship, providing a comprehensive overview of the literature and highlighting areas for future research.
CONCLUSION
In patients with cranial nerve palsies attributed to intracranial aneurysms, the location and rupture status of the aneurysm could determine the type and severity of the nerve palsy. Most of the patients experienced favorable outcomes of the cranial nerve palsies in terms of their resolution and long-term function of the CNP after treatment of the culprit intracranial aneurysms.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Gross is a consultant for Medtronic, Stryker, and MicroVention. Dr. Nogueira reports consulting fees for advisory roles with Stryker Neurovascular, Cerenovus, Medtronic, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals, Imperative Care and stock options for advisory roles with Brainomix, Viz-AI, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte, and Cerebrotech.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We acknowledge Ahmed Muthana for providing the graphical illustration [Figure 2].
References
1. Al-Khayat H, Al-Khayat H, Beshay J, Manner D, White J. Vertebral artery-posteroinferior cerebellar artery aneurysms: Clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery. 2005. 56: 2-11
2. Bartleson JD, Trautmann JC, Sundt TM. Minimal oculomotor nerve paresis secondary to unruptured intracranial aneurysm. Arch Neurol. 1986. 43: 1015-20
3. Bhatti MT, Peters KR, Firment C, Mericle RA. Delayed exacerbation of third nerve palsy due to aneurysmal regrowth after endovascular coil embolization. J Neuroophthalmol. 2004. 24: 3-10
4. Boulouis G, Soize S, Maus V, Fischer S, Lobsien D, Klisch J. Flow diversion for internal carotid artery aneurysms with compressive neuro-ophthalmologic symptoms: Clinical and anatomical results in an international multicenter study. J Neurointerv Surg. 2022. 14: 1090-5
5. Brigui M, Chauvet D, Clarençon F, Degos V, Sourour NA, Nouet A. Recovery from oculomotor nerve palsy due to posterior communicating artery aneurysms: Results after clipping versus coiling in a single-center series. Acta Neurochir. 2014. 156: 879-84
6. Brown BL, Lopes D, Miller DA, Tawk RG, Brasiliense LB, Ringer A. The fate of cranial neuropathy after flow diversion for carotid aneurysms. J Neurosurg. 2016. 124: 1107-13
7. Burkhardt JK, Winkler EA, Lasker GF, Yue JK, Lawton MT. Isolated abducens nerve palsy associated with subarachnoid hemorrhage: A localizing sign of ruptured posterior inferior cerebellar artery aneurysms. J Neurosurg. 2017. 128: 1830-8
8. Chaboki H, Patel AB, Freifeld S, Urken ML, Som PM. Cavernous carotid aneurysm presenting with epistaxis. Head Neck. 2004. 26: 741-6
9. Collins TE, Mehalic TF, White TK, Pezzuti RT. Trochlear nerve palsy as the sole initial sign of an aneurysm of the superior cerebellar artery. Neurosurgery. 1992. 30: 258-61
10. Day AL. Aneurysms of the ophthalmic segment: A clinical and anatomical analysis. J Neurosurg. 1990. 72: 677-91
11. De San Pedro JR. Posterior communicating artery aneurysms causing facial pain: A comprehensive review. Clin Neurol Neurosurg. 2017. 160: 59-68
12. Dimopoulos VG, Fountas KN, Feltes CH, Robinson JS, Grigorian AA. Literature review regarding the methodology of assessing third nerve paresis associated with non-ruptured posterior communicating artery aneurysms. Neurosurg Rev. 2005. 28: 256-60
13. Eddleman CS, Hurley MC, Bendok BR, Batjer HH. Cavernous carotid aneurysms: To treat or not to treat?. Neurosurg Focus. 2009. 26: E4
14. Ferguson GG, Drake CG. Carotid-ophthalmic aneurysms: Visual abnormalities in 32 patients and the results of treatment. Surg Neurol. 1981. 16: 1-8
15. Fujita A, Tamaki N, Yasuo K, Nagashima T, Ehara K. Complete penetration of the optic chiasm by an unruptured aneurysm of the ophthalmic segment: Case report. Surg Neurol. 2002. 57: 130-4
16. Garg K, Singh PK, Mahapatra AK, Sharma BS. Bilateral abducens nerve palsy associated with subarachnoid hemorrhage. Br J Neurosurg. 2014. 28: 771-5
17. Gelener P, Akpinar H. Unruptured aneurysm producing thunderclap headache treated with endovascular coil embolization. Agri. 2018. 30: 146-9
18. Grunwald L, Sund NJ, Volpe NJ. Pupillary sparing and aberrant regeneration in chronic third nerve palsy secondary to a posterior communicating artery aneurysm. Br J Ophthalmol. 2008. 92: 715-6
19. Hall S, Sadek AR, Dando A, Grose A, Dimitrov BD, Millar J. The resolution of oculomotor nerve palsy caused by unruptured posterior communicating artery aneurysms: A cohort study and narrative review. World Neurosurg. 2017. 107: 581-7
20. Hassan T, Hamimi A. Successful endovascular management of brain aneurysms presenting with mass effect and cranial nerve palsy. Neurosurg Rev. 2013. 36: 87-97
21. He W, Gandhi CD, Quinn J, Karimi R, Prestigiacomo CJ. True aneurysms of the posterior communicating artery: A systematic review and meta-analysis of individual patient data. World Neurosurg. 2011. 75: 64-72
22. Howick J. The Oxford 2011 levels of evidence. Available from: http://www.cebm.net/index.aspx?.o=5653 [Last accessed on 2024 Jun 26].
23. Hyland HH, Barnett HJ. The pathogenesis of cranial nerve palsies associated with intracranial aneurysms. Trans Am Neurol Assoc. 1953. 3: 127-31
24. Kameda-Smith M, Pai A, Jung Y, Khan D, Adile AA, Hui K. Third nerve palsy due to intracranial aneurysms and recovery after endovascular coiling. Can J Neurol Sci. 2022. 49: 560-8
25. Kang SD. Ruptured anterior communicating artery aneurysm causing bilateral oculomotor nerve palsy: A case report. J Korean Med Sci. 2007. 22: 173-6
26. Kasner SE, Liu GT, Galetta SL. Neuro-ophthalmologic aspects of aneurysms. Neuroimaging Clin N Am. 1997. 7: 679-92
27. Kassis SZ, Jouanneau E, Tahon FB, Salkine F, Perrin G, Turjman F. Recovery of third nerve palsy after endovascular treatment of posterior communicating artery aneurysms. World Neurosurg. 2010. 73: 11-6
28. Kerns JM, Smith DR, Jannotta FS, Alper MG. Oculomotor nerve regeneration after aneurysm surgery. Am J Ophthalmol. 1979. 87: 225-33
29. Kobets AJ, Scoco A, Nakhla J, Brook AL, Kinon MD, Baxi N. Flow-diverting stents for the obliteration of symptomatic, infectious cavernous carotid artery aneurysms. Oper Neurosurg. 2018. 14: 681-5
30. Kono K, Shintani A, Okada H, Tanaka Y, Terada T. Stent-assisted coil embolization for cavernous carotid artery aneurysms. Neurol Med Chir (Tokyo). 2014. 54: 126-32
31. Koskela E, Laakso A, Kivisaari R, Setälä K, Hijazy F, Hernesniemi J. Eye movement abnormalities after a ruptured intracranial aneurysm. World Neurosurg. 2015. 83: 362-7
32. Koskela E, Setälä K, Kivisaari R, Hernesniemi J, Laakso A. Neuro-ophthalmic presentation and surgical results of unruptured intracranial aneurysms-prospective Helsinki experience of 142 patients. World Neurosurg. 2015. 83: 614-9
33. Kraus RR, Kattah J, Bortolotti C, Lanzino G. Oculomotor palsy from an unruptured posterior communicating artery aneurysm presenting with cerebrospinal fluid pleocytosis and enhancement of the third cranial nerve: Case report. J Neurosurg. 2004. 101: 352-3
34. Kurokawa R, Saito R, Nakamura Y, Kagami H, Ichikizaki K. Ruptured vertebral artery-posterior inferior cerebellar artery aneurysm associated with facial nerve paresis successfully treated with interlocking detachable coils-case report. Neurol Med Chir. 1999. 39:
35. Kurokawa Y, Ishizaki E, Inaba KI. Incomplete oculomotor nerve palsy caused by an unruptured internal carotid-anterior choroidal artery aneurysm-case report. Neurol Med Chir. 2005. 45: 143-7
36. Kyriakides T, Aziz TZ, Torrens MJ. Postoperative recovery of third nerve palsy due to posterior communicating aneurysms. Br J Neurosurg. 1989. 3: 109-11
37. Lehto H, Niemelä M, Kivisaari R, Laakso A, Jahromi BR, Hijazy F. Intracranial vertebral artery aneurysms: Clinical features and outcome of 190 patients. World Neurosurg. 2015. 84: 380-9
38. Lownie SP, Drake CG, Peerless SJ, Ferguson GG, Pelz DM. Clinical presentation and management of giant anterior communicating artery region aneurysms. J Neurosurg. 2000. 92: 267-77
39. Lylyk P, Ceratto R, Hurvitz D, Basso A. Treatment of a vertebral dissecting aneurysm with stents and coils: Technical case report. Neurosurgery. 1998. 43: 385-8
40. Maroon JC, Lunsford LD, Deeb ZL. Hemifacial spasm due to aneurysmal compression of the facial nerve. Arch Neurol. 1978. 35: 545-6
41. Martinez-Perez R, Tsimpas A, Joswig H, Hernandez-Alvarez V, Mura J. Extradural minipterional approach for giant intracranial aneurysms. Surg Neurol Int. 2020. 11: 382
42. Matano F, Murai Y, Mizunari T, Tamaki T, Tateyama K, Koketsu K. Recovery of visual and ophthalmologic symptoms after treating large or giant internal carotid artery aneurysm by high-flow bypass with cervical ligation. World Neurosurg. 2017. 98: 182-8
43. Menghini VV, Brown RD, Sicks JD, O’Fallon WM, Wiebers DO. Clinical manifestations and survival rates among patients with saccular intracranial aneurysms: Population-based study in Olmsted County, Minnesota, 1965 to 1995. Neurosurgery. 2001. 49: 251-8
44. Miyazawa T, Uozumi Y, Tsuzuki N, Shima K. “Phosphene”: Early sign of vascular compression neuropathy of the optic nerve. Acta Neurochir. 2009. 151: 1315-7
45. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, editors. Systematic reviews of etiology and risk. Joanna Briggs Institute reviewer’s manual. Adelaide, Australia: The Joanna Briggs Institute; 2017. p. 217-69
46. Moon K, Albuquerque FC, Ducruet AF, Crowley RW, McDougall CG. Resolution of cranial neuropathies following treatment of intracranial aneurysms with the Pipeline Embolization Device. J Neurosurg. 2014. 121: 1085-92
47. Morishita H, Nakamura S, Toma N, Nakatsuka Y, Takeuchi K. A case of vertebral artery aneurysm presenting with dysphagia. Auris Nasus Larynx. 2017. 44: 479-83
48. Murakami H, Kawaguchi T, Fukuda M, Ito Y, Hasegawa H, Tanaka R. Monitoring of the lateral spread response in the endovascular treatment of a hemifacial spasm caused by an unruptured vertebral artery aneurysm: Case report. J Neurosurg. 2004. 101: 861-3
49. Nam KH, Choi CH, Lee JI, Ko JG, Lee TH, Lee SW. Unruptured intracranial aneurysms with oculomotor nerve palsy: Clinical outcome between surgical clipping and coil embolization. J Korean Neurosurg Soc. 2010. 48: 109-14
50. Nathal E, Degollado-García J, Bonilla-Suastegui A, RodríguezRubio HA, Ferrufino-Mejia BR, Casas-Martínez MR. Microsurgical treatment of a giant intracavernous carotid artery aneurysm in a pediatric patient: Case report and literature review. Cureus. 2023. 15: e34010
51. Nathal E, Yasui N, Suzuki A, Hadeishi H. Ruptured anterior communicating artery aneurysm causing bilateral a bducens nerve paralyses-case report. Neurol Med Chir. 1992. 32: 17-20
52. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
53. Park JH, Kim TH, Shin JJ, Shin HS, Hwang YS. Anterior communicating artery aneurysm related to visual symptoms. J Korean Neurosurg Soc. 2009. 46: 232-8
54. Parr M, Carminucci A, Al-Mufti F, Roychowdhury S, Gupta G. Isolated abducens nerve palsy associated with ruptured posterior inferior cerebellar artery aneurysm: Rare neurologic finding. World Neurosurg. 2019. 121: 97-9
55. Patel K, Guilfoyle MR, Bulters DO, Kirollos RW, Antoun NM, Higgins JN. Recovery of oculomotor nerve palsy secondary to posterior communicating artery aneurysms. Br J Neurosurg. 2014. 28: 483-7
56. Peerless S, Drake C, editors. Management of aneurysms of the posterior circulation. Neurological surgery. Philadelphia, PA: W.B. Saunders Co.; 1990. 3: 1764-806
57. Rodriguez-Catarino M, Frisén L, Wikholm G, Elfverson J, Quiding L, Svendsen P. Internal carotid artery aneurysms, cranial nerve dysfunction and headache: The role of deformation and pulsation. Neuroradiology. 2003. 45: 236-40
58. Santillan A, Zink WE, Knopman J, Riina HA, Gobin YP. Early endovascular management of oculomotor nerve palsy associated with posterior communicating artery aneurysms. Interv Neuroradiol. 2010. 16: 17-21
59. Sarwar M. Abducens nerve paralysis due to giant aneurysm in the medial carotid canal: Case report. J Neurosurg. 1977. 46: 121-3
60. Sgreccia A, Caragliano A, Sanfilippo G, Campa S, Trignani R, Giannoni M. Rare and symptomatic cavernous donut-shaped aneurysm treated by flow diverter deployment. World Neurosurg. 2019. 121: 227-31
61. Signorelli F, Pop R, Ganau M, Cebula H, Scibilia A, Gallinaro P. Endovascular versus surgical treatment for improvement of oculomotor nerve palsy caused by unruptured posterior communicating artery aneurysms. J Neurointerv Surg. 2020. 12: 964-7
62. Silva MN, Saeki N, Hirai S, Yamaura A. Unusual cranial nerve palsy caused by cavernous sinus aneurysms: Clinical and anatomical considerations reviewed. Surg Neurol. 1999. 52: 143-9
63. Sobol EK, Carter KL, Ibrahim K, Alfaro C, Patel E, Pasquale LR. A convergence of ophthalmic and life-threatening emergencies: Acute angle closure glaucoma and subarachnoid hemorrhage. J Glaucoma. 2019. 28: e151-2
64. Stiebel-Kalish H, Kalish Y, Bar-On RH, Setton A, Niimi Y, Berenstein A. Presentation, natural history, and management of carotid cavernous aneurysms. Neurosurgery. 2005. 57: 850-7
65. Sudhoff H, Stark T, Knorz S, Luckhaupt H, Borkowski G. Massive epistaxis after rupture of intracavernous carotid artery aneurysm: Case report. Ann Otol Rhinol Laryngol. 2000. 109: 776-8
66. Suzuki N, Suzuki M, Araki S, Sato H. A case of multiple cranial nerve palsy due to sphenoid sinusitis complicated by cerebral aneurysm. Auris Nasus Larynx. 2005. 32: 415-9
67. Tajima A, Ito M, Ishii M. Complete recovery from monocular blindness caused by aneurysmal compression to optic nerve-report of two cases. Neurol Med Chir. 1993. 33: 19-23
68. Takahashi M, Kase M, Suzuki Y, Yokoi M, Kazumata K, Terasaka S. Incomplete oculomotor palsy with pupil sparing caused by compression of the oculomotor nerve by a posterior communicating posterior cerebral aneurysm. Jpn J Ophthalmol. 2007. 51: 470-3
69. Tan H, Huang G, Zhang T, Liu J, Li Z, Wang Z. A retrospective comparison of the influence of surgical clipping and endovascular embolization on recovery of oculomotor nerve palsy in patients with posterior communicating artery aneurysms. Neurosurgery. 2015. 76: 687-94
70. Teasdale E, Statham P, Straiton J, Macpherson P. Non-invasive radiological investigation for oculomotor palsy. J Neurol Neurosurg Psychiatry. 1990. 53: 549-53
71. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018. 169: 467-73
72. Tsuboi K, Shibuya F, Yamada T, Nose T. Giant aneurysm at the junction of the left internal carotid and persistent primitive trigeminal arteries-case report. Neurol Med Chir. 1992. 32: 778-81
73. Tummala RP, Harrison A, Madison MT, Nussbaum ES. Pseudomyasthenia resulting from a posterior carotid artery wall aneurysm: A novel presentation: Case report. Neurosurgery. 2001. 49: 1466-9
74. Weber W, Siekmann R, Kis B, Kuehne D. Treatment and follow-up of 22 unruptured wide-necked intracranial aneurysms of the internal carotid artery with Onyx HD 500. Am J Neuroradiol. 2005. 26: 1909-15
75. Wong GK, Boet R, Poon WS, Yu S, Lam JM. A review of isolated third nerve palsy without subarachnoid hemorrhage using computed tomographic angiography as the first line of investigation. Clin Neurol Neurosurg. 2004. 107: 27-31
76. Yasargil M, editors. Carotid-ophthalmic aneurysms. Microneurosurgery: Clinical considerations, surgery of the intracranial aneurysms and results. Stuttgart: Thieme; 1984. 2: 43-55
77. Yi JL, Galgano MA, Tovar-Spinoza Z, Deshaies EM. Coil embolization of an intracranial aneurysm in an infant with tuberous sclerosis complex: A case report and literature review. Surg Neurol Int. 2012. 3: 129
78. Zelman S, Goebel MC, Manthey DE, Hawkins S. Large posterior communicating artery aneurysm: Initial presentation with reproducible facial pain without cranial nerve deficit. West J Emerg Med. 2016. 17: 808-10
79. Zhang SH, Pei W, Cai XS, Cheng G. Endovascular management and recovery from oculomotor nerve palsy associated with aneurysms of the posterior communicating artery. World Neurosurg. 2010. 74: 316-9
80. Zheng F, Chen X, Zhou J, Pan Z, Xiong Y, Huang X. Clipping versus coiling in the treatment of oculomotor nerve palsy induced by unruptured posterior communicating artery aneurysms: A meta-analysis of cohort studies. Clin Neurol Neurosurg. 2021. 206: 106689
81. Zhong W, Zhang J, Shen J, Zhang P, Wang D, Su W. Posterior communicating aneurysm with oculomotor nerve palsy: Predictors of nerve recovery. J Clin Neurosci. 2019. 59: 62-7