- Department of Neurosurgery, Mental Health and Neuroscience, Maastricht University Medical Center, Maastricht, The Netherlands
- Division of Neuroanatomy, Department of Anatomy, King Khalid University, Abha, Saudi Arabia
- Division of Neurosurgery, Department of Surgery, King Khalid University, Abha, Saudi Arabia
- European Graduate School of Neuroscience (EURON), Maastricht University, Maastricht, The Netherlands
Correspondence Address:
Sarah Hescham
Department of Neurosurgery, Mental Health and Neuroscience, Maastricht University Medical Center, Maastricht, The Netherlands
DOI:10.4103/sni.sni_342_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Majed Aldehri, Yasin Temel, Ibrahim Alnaami, Ali Jahanshahi, Sarah Hescham. Deep brain stimulation for Alzheimer's Disease: An update. 07-Mar-2018;9:58
How to cite this URL: Majed Aldehri, Yasin Temel, Ibrahim Alnaami, Ali Jahanshahi, Sarah Hescham. Deep brain stimulation for Alzheimer's Disease: An update. 07-Mar-2018;9:58. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8805
Abstract
Background:Dementia is among the leading causes of severe and long-term disability worldwide, decreasing the quality of life of individuals and families. Moreover, it induces an enormous economic burden on societies. The most prevalent cause of dementia is Alzheimer's disease (AD). Because current treatment options for AD are limited, deep brain stimulation (DBS) has been considered.
Methods:The aim of this review is to survey the current understanding regarding the effects of DBS in AD and possibly shed light on the mechanisms of DBS in AD. We searched PubMed and Cochrane for various studies in English literature describing DBS in patients with AD and relevant preclinical studies. All related studies published from December 2013 to March 2017 were included in this review.
Results:Our understanding of the neural circuitry underlying learning and memory in both rodent models and human patients has grown over the past years and provided potential therapeutic targets for DBS such as the fornix and the nucleus basalis of Meynert. Clinical results indicate that DBS is most beneficial for patients who are in the early stages of AD. Potential mechanisms of action of DBS in AD comprise long-term structural plasticity, including hippocampal enlargement as well as enhanced neurotransmitter release.
Conclusion:It is still premature to conclude that DBS can be used in the treatment of AD, and the field will wait for the results of ongoing and future clinical trials.
Keywords: Alzheimer's disease, deep brain stimulation, dementia, memory, fornix, nucleus basalis of Meynert
INTRODUCTION
Patients with dementia suffer from progressive cognitive decline. The most prevalent cause of dementia is Alzheimer's disease (AD) as it constitutes 50–80% of the cases. Dementia is a serious socioeconomic threat for ageing societies.[
One of the neuromodulation approaches considered for AD is deep brain stimulation (DBS).[
Previously, we have reviewed relevant studies, which have been published until 2013.[
In the last few years, new data has appeared on DBS in AD [
LITERATURE SEARCH
We searched PubMed and Cochrane library for various clinical and preclinical studies in English literature with the search terms “Deep Brain Stimulation,” “memory loss,” “cognitive impairment,” “dementia,” and “Alzheimer's Disease.” Key words were used independently and in different combinations. Relevant articles were chosen from review papers, original research articles, and book chapters about DBS and AD. Articles of interest within the reference lists of selected articles were also considered. Studies describing DBS in AD patients using the fornix or NBM as the target structure were included. Clinical outcomes were Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-cog), Mini-Mental State Examination (MMSE), and/or Clinical Dementia Rating Scale Sum of Boxes (CDR-SB). Preclinical studies targeting the fornix, NBM, or different thalamic nuclei were also included. Outcome measures were performance in behavioral tests (e.g. Morris water maze and fear conditioning). Articles aimed to study the effect of DBS in other neurodegenerative diseases or other forms of dementia [e.g. Parkinson's Disease dementia, vascular dementia, Huntington's disease dementia, alcohol related dementia, Creutzfeldt–Jakob disease, Lewy-body dementia] were excluded. Moreover, case reports and articles written in languages other than English were also excluded. To update our previous review,[
CLINICAL STUDIES
Thus far, only two different brain targets have been implicated for DBS in AD patients. These targets include the fornix[
As described in our previous review, the idea of using fornix DBS for memory restoration was found incidentally by Hamani et al.[
Glucose metabolism also changed in DBS patients in accordance with volume changes of the hippocampus and mammillary bodies. Deformation-based morphometry (DBM) showed local enlargement in regions that are typically atrophied in AD patients such as parahippocampal gyri, right superior temporal gyrus, left parietal lobule, and bilateral precuneus.[
Following the abovementioned phase I trial, Lozano and colleagues launched the ADvance study, a double-blinded randomized controlled study in which 42 AD patients were implanted with fornix DBS electrodes in different centers across the U.S. and Canada. The aim of the study was to investigate the long and short-term safety of DBS. Outcome measures were neuropsychological tests such as the ADAS-cog, clinical dementia ratings, and glucose metabolism.[
The outcomes of the phase II trial of the ADvance study have recently been published.[
Another potential target for DBS in AD patients is the NBM because it has cholinergic projections to hippocampus and neocortex. In AD, NBM degenerates, which results in reduction of cholinergic transmission, and subsequently decline of cognition in patients.[
Recently, 6 mild-moderate AD patients were enrolled in a German study and received bilateral low frequency DBS of the NBM.[
In a next publication, the nutritional status of these patients was assessed before receiving NBM DBS and after 1 year to analyze potential associations between changes in cognition and nutritional status.[
The same group performed a follow-up study in 2 patients, different from the 6 patients recruited in the pilot study based on the hypothesis that earlier intervention results in a better outcome. They targeted the same location (NBM) in 2 younger patients (61 and 67 years) with an earlier stage of AD.[
PRECLINICAL STUDIES
Different brain targets have been implicated for DBS in animal models which showed enhancement of memory functions. These sites include the fornix,[
Recently, the effects of fornix DBS in a mouse model of Rett syndrome has been evaluated.[
Interestingly, in the same year, a neurochemical study was published in which the effects of fornix DBS with regard to hippocampal neurotransmitter release was described.[
With regard to neurochemical effects of NBM stimulation in rats with basal forebrain cholinergic neurons degeneration, gamma-aminobutyric acid (GABA), and glutamate seem to play a role in restoring memory loss.[
Since 2013, various animal studies have been published investigating DBS in different thalamic nuclei. In line with this, DBS of the rostral intralaminar thalamic nucleus (ILN) improved the acquisition of spatial memory when compared to sham and control rats.[
In another study, the effect of biphasic unilateral stimulation of the anteromedial thalamic nucleus on neurogenesis was investigated in awake and unrestrained rats.[
Recently, DBS of the parafascicular thalamic nucleus has shown to affect NMDA receptor GluN1 subunit gene expression in the prefrontal cortex.[
DISCUSSION
Our understanding of the neural circuitry underlying learning and memory in both rodent models and human patients has grown over the past years and provided us with potential therapeutic targets for DBS. Phase I trials of DBS for AD, targeting either the fornix[
Furthermore, an increasing amount of preclinical studies aim to elucidate underlying mechanisms of action [
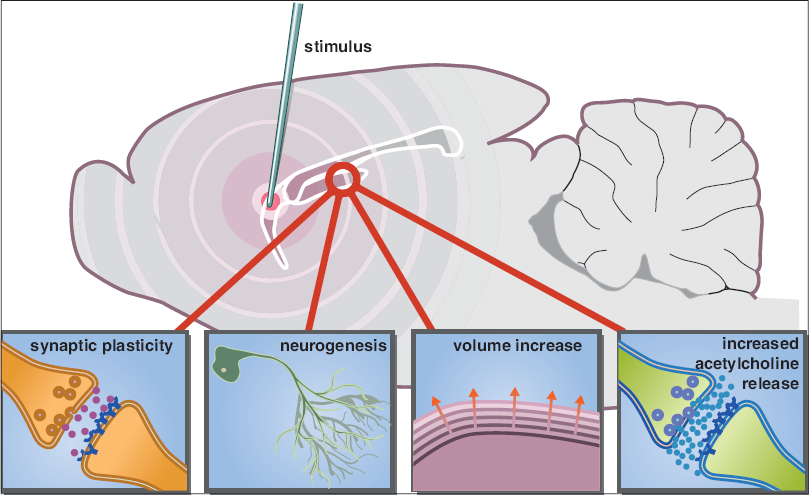
Figure 1
An updated schematic representation of the potential mechanisms involved in enhancing memory functions by deep brain stimulation. Stimulation of a target area within the memory circuit (e.g. fornix) can modulate the hippocampus through synaptic plasticity, neurogenesis, volume increase, and increased acetylcholine release
A cost-effectiveness study found that the clinical and economic thresholds required for DBS to be considered cost-effective for AD are relatively low.[
Although the discussed therapeutics have shown promise in improving memory in AD, it is important to acknowledge that AD patients suffer from overall cognitive deterioration as well as non-cognitive symptoms and not solely memory impairment. Therefore, it is difficult to determine if improving memory will enhance quality of life. Moreover, it should be noted, that DBS does not prevent neurodegeneration in AD. Symptoms are only alleviated temporarily and in specific patient groups. Studies have shown that improvement in cognitive functioning and memory can last for a few years and deterioration can occur afterwards. In addition, it remains unclear which inclusion criteria should be considered for AD patients to benefit from DBS. With regard to the age of the patients, it is possible that the cognitive decline in young AD patients is related to DBS itself as has been observed in some PD cases.[
Nevertheless, because the clinical burden of dementia is considerable and the efficacy of current medical treatments limited, further investigation is warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Association As. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011. 7: 208-
2. Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009. 30: 1711-23
3. Bartus RT, Dean Rr, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982. 217: 408-414
4. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Pub Health. 1998. 88: 1337-42
5. Chamaa F, Sweidan W, Nahas Z, Saade N, Abou-Kheir W. Thalamic Stimulation in Awake Rats Induces Neurogenesis in the Hippocampal Formation. Brain Stimulat. 2016. 9: 101-8
6. Fernández-Cabrera MR, Selvas A, Miguéns M, Higuera-Matas A, Vale-Martínez A, Ambrosio E. Parafascicular thalamic nucleus deep brain stimulation decreases NMDA receptor GluN1 subunit gene expression in the prefrontal cortex. Neuroscience. 2017. 348: 73-82
7. Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012. 8: 189-202
8. Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009. 66: 781-5
9. Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008. 63: 119-23
10. Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol. 2011. 232: 100-4
11. Hao S, Tang B, Wu Z, Ure K, Sun Y, Tao H. Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature. 2015. 526: 430-4
12. Hescham S, Jahanshahi A, Schweimer JV, Mitchell SN, Carter G, Blokland A. Fornix deep brain stimulation enhances acetylcholine levels in the hippocampus. Brain Struct Funct. 2016. 221: 4281-6
13. Hescham S, Lim LW, Jahanshahi A, Blokland A, Temel Y. Deep brain stimulation in dementia-related disorders. Neurosci Biobehav Rev. 2013. 37: 2666-75
14. Hescham S, Lim LW, Jahanshahi A, Steinbusch HW, Prickaerts J, Blokland A. Deep brain stimulation of the forniceal area enhances memory functions in experimental dementia: The role of stimulation parameters. Brain Stimulat. 2013. 6: 72-77
15. Hotta H, Kagitani F, Kondo M, Uchida S. Basal forebrain stimulation induces NGF secretion in ipsilateral parietal cortex via nicotinic receptor activation in adult, but not aged rats. Neurosci Res. 2009. 63: 122-8
16. Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med. 2010. 16: 1210-4
17. Kahle-Wrobleski K, Ye W, Henley D, Hake AM, Siemers E, Chen Y-F. Assessing quality of life in Alzheimer's disease: Implications for clinical trials. Alzheimers Dement. 2017. 6: 82-90
18. Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's dementia. Mol Psychiatry. 2015. 20: 353-60
19. Kuhn J, Hardenacke K, Shubina E, Lenartz D, Visser-Vandewalle V, Zilles K. Deep Brain Stimulation of the Nucleus Basalis of Meynert in Early Stage of Alzheimer's Dementia. Brain Stimulat. 2015. 8: 838-9
20. Kuhn J, Hardenacke K, Shubina E, Lenartz D, Visser-Vandewalle V, Zilles K. Deep Brain Stimulation of the Nucleus Basalis of Meynert in Early Stage of Alzheimer's Dementia. Brain Stimul. 2015. 8: 838-9
21. Laxton AW, Lozano AM. Deep brain stimulation for the treatment of Alzheimer disease and dementias. World Neurosurg. 2013. 80: S28 e21-28
22. Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010. 68: 521-34
23. Lee JE, Jeong DU, Lee J, Chang WS, Chang JW. The effect of nucleus basalis magnocellularis deep brain stimulation on memory function in a rat model of dementia. BMC Neurol. 2016. 16: 1-
24. Lobo A, Launer L, Fratiglioni L, Andersen K, Di Carlo A, Breteler M. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology. 2000. 54: S4-9
25. Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos J-M, Munro C, Oh E. A phase II study of fornix deep brain stimulation in mild Alzheimer's disease. J Alzheimers Dis. 2016. 54: 777-87
26. Massano J, Garrett C. Deep brain stimulation and cognitive decline in Parkinson's disease: A clinical review. Front Neurol. 2012. 3: 66-
27. Mirsaeedi-Farahani K, Halpern CH, Baltuch GH, Wolk DA, Stein SC. Deep brain stimulation for Alzheimer disease: A decision and cost-effectiveness analysis. J Neurol. 2015. 262: 1191-7
28. Mirzadeh Z, Bari A, Lozano AM. The rationale for deep brain stimulation in Alzheimer's disease. J Neural Transm (Vienna). 2016. 123: 775-83
29. Noreik M, Kuhn J, Hardenacke K, Lenartz D, Bauer A, Bührle CP. Changes in nutritional status after deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's disease – Results of a phase I study. J Nutr Health Aging. 2015. 19: 812-8
30. Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007. 29: 125-32
31. Ponce FA, Asaad WF, Foote KD, Anderson WS, Rees Cosgrove G, Baltuch GH. Bilateral deep brain stimulation of the fornix for Alzheimer's disease: Surgical safety in the ADvance trial. J Neurosurg. 2016. 125: 75-84
32. Sankar T, Chakravarty MM, Bescos A, Lara M, Obuchi T, Laxton AW. Deep brain stimulation influences brain structure in Alzheimer's disease. Brain Stimulat. 2015. 8: 645-54
33. Sos-Hinojosa H, Guillazo-Blanch G, Vale-MartÍnez A, Nadal R, Morgado-Bernal I, MartÍ-Nicolovius M. Parafascicular electrical stimulation attenuates nucleus basalis magnocellularis lesion-induced active avoidance retention deficit. Behav Brain Res. 2003. 144: 37-48
34. Stone SS, Teixeira CM, DeVito LM, Zaslavsky K, Josselyn SA, Lozano AM. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011. 31: 13469-84
35. Temel Y, Jahanshahi A. Treating brain disorders with neuromodulation. Science. 2015. 347: 1418-9
36. Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008. 108: 132-8
37. Tsai S-T, Chen L-J, Wang Y-J, Chen S-Y, Tseng G-F. Rostral Intralaminar Thalamic Deep Brain Stimulation Triggered Cortical and Hippocampal Structural Plasticity and Enhanced Spatial Memory. Stereotact Funct Neurosurg. 2016. 94: 108-17
38. Varma VR, Chuang Y-f, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2015. 25: 605-15
39. Wilson RS, Boyle PA, Yu L, Barnes LL, Sytsma J, Buchman AS. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015. 85: 984-91