- Professor of Clinical Neurosurgery, School of Medicine, State University of N.Y. at Stony Brook, New York, USA
- Chief of Neurosurgical Spine and Education NYU Winthrop Hospital, NYU Winthrop NeuroScience, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Professor of Clinical Neurosurgery, School of Medicine, State University of N.Y. at Stony Brook, New York, USA
Chief of Neurosurgical Spine and Education NYU Winthrop Hospital, NYU Winthrop NeuroScience, Mineola, New York, USA
DOI:10.4103/sni.sni_208_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Definitions and treatments for chiari-1 malformations and its variants: Focused review. 27-Jul-2018;9:152
How to cite this URL: Nancy E. Epstein. Definitions and treatments for chiari-1 malformations and its variants: Focused review. 27-Jul-2018;9:152. Available from: http://surgicalneurologyint.com/surgicalint-articles/definitions-and-treatments-for-chiari%e2%80%911-malformations-and-its-variants-focused-review/
Abstract
Background:Reviewing the neurosurgical literature demonstrated that spinal neurosurgeons rarely (0.78%) diagnose chiari-1 malformation (CM-1) in adults on magnetic resonance (MR) studies defined by tonsillar descent >5 mm below the foramen magnum (FM). Children, averaging 10 years of age, exhibit CM-1 in 96/100,000 cases. According to the literature, fewer spinal neurosurgeons additionally recognize and treat the low lying cerebellar tonsil (LLCT) syndrome.
Methods:The normal location of the cerebellar tonsils on cranial/cervical MR averages 2.9 mm ± 3.4 mm above or up to 3 mm below the FM. The neurosurgical literature revealed that most neurosurgeons diagnose and treat CM-1 where the tonsils are >5 mm to an average of 12 mm below the FM. Fewer spinal neurosurgeons additionally diagnose and treat the LLCT syndrome defined by
Results:According to the neurosurgical literature, many neurosurgeons perform cranial/spinal decompression with/without fusion and/or duraplasty for CM-1. Fewer neurosurgeons perform these procedures for CM-1 and the LLCT syndrome, for which they additionally perform preoperative cervical traction under anesthesia, and the postoperative placement of occipital neurostimulators (ONS) for intractable headaches following chiari-1/LLCT surgery.
Conclusion:Reviewing the literature revealed that spinal neurosurgeons rarely diagnose CM-1, and treat them with decompressions with/without fusions and/or duraplasty. Fewer spinal neurosurgeons diagnose/treat both the CM-1 and LLCT syndromes, perform preoperative traction under anesthesia, and place ONS for persistent headaches following CM-1 surgery.
Keywords: Chiari-1 malformations (CM-1): low lying cerebellar tonsil syndrome, chiari-1 surgery, occipital neurostimulators, surgical indications, traction under anesthesia
INTRODUCTION
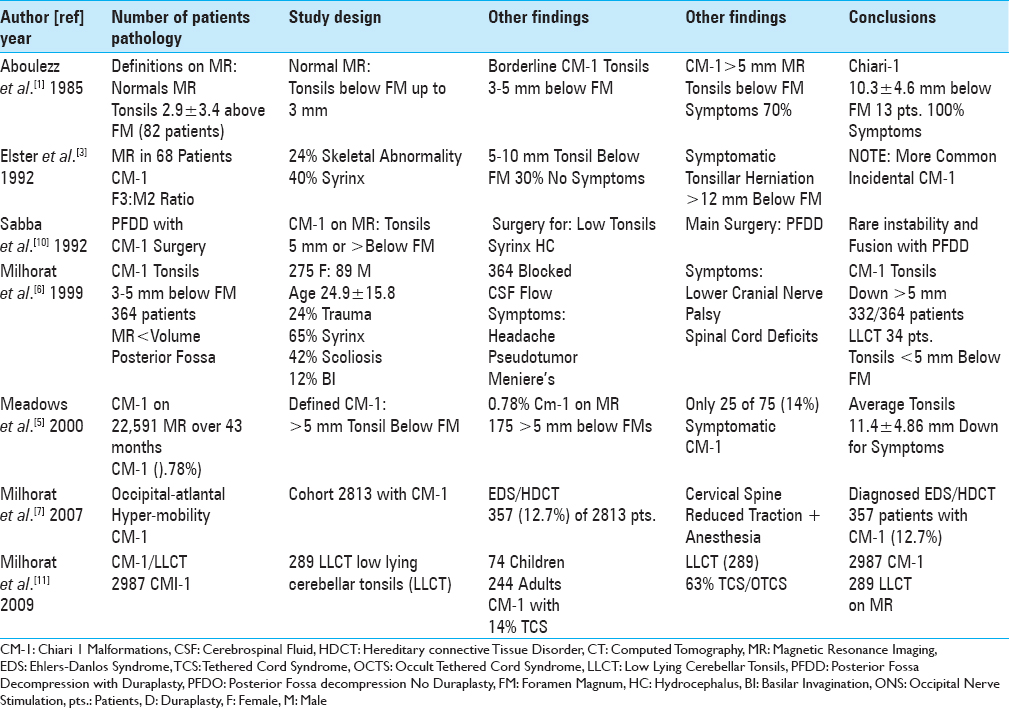
A review of the neurosurgical literature revealed that spinal neurosurgeons rarely (0.78%) diagnose chiari-1 malformations (CM-1) on magnetic resonance (MR) studies [Tables
Table 2
According to a review of the neurosurgical literature, spinal neurosurgeons typically defined chiari-1 malformations by tonsillar descent >5 mm below the foramen magnum vs. while fewer spinal neurosurgeons additionally defned the low lying cerebellar tonsil syndrome (LLCT:< 5 mm tonsillar descent) 2011-2018
The literature also showed that fewer spinal neurosurgeons additionally diagnose and treat the low lying cerebellar tonsil syndrome (LLCT) defined on MR by tonsils herniated <5 mm below the foramen magnum [Tables
Here, we reviewed the diagnosis of the CM-1 and LLCT sydromes, and highlighted the different therapeutic options offered by spinal neurosurgeons based on a review of the neurosurgical literature.
Frequency of chiari-1 malformations (CM-1) diagnosed in the adult and pediatric populations
The frequency of CM-1 malformations is low in both the adult and pediatric age groups. In 2000, when Meadows et al. reviewed 22,591 MR studies performed over a 43-month period, they found only 0.78% (175 patients) of patients exhibited CM-1 malformations (e.g. defined by tonsils > 5 mm below the foramen magnum) [
Classical definition of chiari-1 malformations (CM-1)
According to a review of the neurosurgical literature, spinal neurosurgeons typically define CM-1 utilizing MR scans that show >5 mm of tonsillar descent [Tables
Extent of tonsillar descent correlates with symptomatic chiari-1 malformations (CM-1)
Different studies correlated the onset of chiari-1 symptoms with the extent of tonsillar descent below the FM [
Fewer spinal neurosurgeons define low lying cerebellar tonsil syndrome (LLCT)
A review of the literature revealed that fewer spinal neurosurgeons additionally diagnosed the LLCT on MR [
Associated abnormalities with adult and pediatric chiari-1 malformation (CM-1)
Multiple additional pathologies accompany CM-1 malformations [Tables
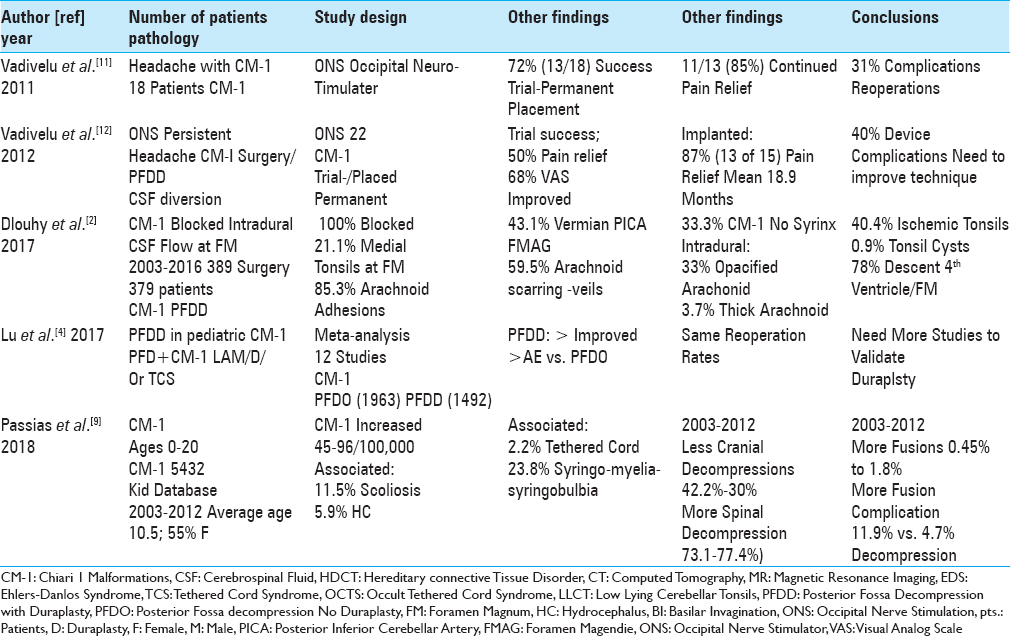
Increased incidence of spinal decompression and fusion rates for chiari-1 malformation (CM-1)
Recently, higher spinal decompression and fusion rates have characterized CM-1 surgery in the pediatric population [
Pros and cons for posterior fossa decompression only or with duraplasty for chiari-1 malformation
There are various pros and cons for performing posterior fossa decompression only (PFDO) vs. posterior fossa decompression with duraplasty (PFDD) for patients with CM-1 [Tables
Literature demonstrates fewer spinal neurosurgeons diagnose Ehlers-Danlos syndrome treated with cervical traction under anesthesia
The literature showed that fewer spinal neurosurgeons performed traction under anesthesia for patients with hereditary connective tissue disorder (HCTD)/Ehlers–Danlos syndrome (EDS) [
Literature shows fewer spinal neurosurgeons perform occipital neurostimulator trials/permanent implants for persistent headache following chiari-1 malformation surgery
A review of the literature showed that fewer spinal neurosurgeons additionally placed ONS for patients with intractable headaches following CM-1 surgery [
CONCLUSION
According to our review of the neurosurgical literature, spinal neurosurgeons typically perform decompressions with/without fusions and/or duraplasty for CM-1 malformations. The literature, however, demonstrated that fewer spinal neurosurgeons additionally diagnosed and treated the LLCT syndrome, for which they also performed postoperative traction under anesthesia, and placed postoperative ONS for persistent headaches following CM-1 surgery (posterio decompressions with/without duraplasty and/or fusions with a 40% complication rate). Future studies should reexamine how spinal neurosurgeons define and treat the LLCT syndrome, the indications for preoperative traction under anesthesia, and the safety/efficacy of ONS placement.
References
1. Aboulezz AO, Sartor K, Geyer CA, Gado MH. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitative approach to MR imaging. J Comput Assist Tomogr. 1985. 9: 1033-6
2. Dlouhy BJ, Dawson JD, Menezes AH. Intradural pathology and pathophysiology associated with Chiari I malformation in children and adults with and without syringomyelia. J Neurosurg Pediatr. 2017. 20: 526-41
3. Elster Ad, Chen MYM. Chiari I malformations; clinical and radiologic reappraisal. Radiology. 1992. 183: 347-53
4. Lu VM, Phan K, Crowley SP, Daniels DJ. The addition of duraplasty to posterior fossa decompression in the surgical treatment of pediatric Chiari malformation Type I: A systematic review and meta-analysis of surgical and performance outcomes. J Neurosurg Pediatr. 2017. 20: 439-49
5. Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000. 92: 920-6
6. Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999. 44: 1005-17
7. Milhorat TH, Bolognese PA, Nishikawa M, McDonnell NB, Francomano CA. Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine. 2007. 7: 601-9
8. Milhorat TH, Bolognese PA, Nishikawa M, Francomano CA, McDonnell NB, Roonprapunt C. Association of Chiari malformation type I and tethered cord syndrome: Preliminary results of sectioning filum terminale. Surg Neurol. 2009. 72: 20-35
9. Passias PG, Pyne A, Horn SR, Poorman GW, Janjua MB, Vasquez-Montes D. Developments in the treatment of Chiari type 1 malformations over the past decade. J Spine Surg. 2018. 4: 45-54
10. Sabba MF, Renor BS, Ghizoni E, Tedeschi H, Joaquim AF. Posterior fossa decompression with duraplasty in Chiari surgery: A technical note. Rev Assoc Med Bras (1992). 2017. 63: 946-9
11. Vadivelu S, Bolognese P, Milhorat TH, Mogilner AY. Occipital neuromodulation for refractory headache in the Chiari malformation population. Prog Neurol Surg. 2011. 24: 118-25
12. Vadivelu S, Bolognese P, Milhorat TH, Mogilner AY. Occipital nerve stimulation for refractory headache in the Chiari malformation population. Neurosurgery. 2012. 70: 1430-6