- Department of Emergency Medicine Yamagata City Hospital, Saiseikan, Nanokamachi, Yamagata, Japan.
- Department of Neurosurgery, Yamagata City Hospital, Saiseikan, Nanokamachi, Yamagata, Japan.
- Department of Neurosurgery, School of Medicine, Yamagata University, Kojirakawamachi, Yamagata, Japan.
Correspondence Address:
Atsushi Kuge
Department of Neurosurgery, School of Medicine, Yamagata University, Kojirakawamachi, Yamagata, Japan.
DOI:10.25259/SNI_529_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Atsushi Kuge1,2, Rei Kondo2, Yuta Mitobe2, Tetsu Yamaki2, Shinji Sato2, Shinjiro Saito2, Yukihiko Sonoda3. Delayed acute subdural hematoma treated with endoscopic procedure: A case report. 21-Oct-2020;11:350
How to cite this URL: Atsushi Kuge1,2, Rei Kondo2, Yuta Mitobe2, Tetsu Yamaki2, Shinji Sato2, Shinjiro Saito2, Yukihiko Sonoda3. Delayed acute subdural hematoma treated with endoscopic procedure: A case report. 21-Oct-2020;11:350. Available from: https://surgicalneurologyint.com/surgicalint-articles/delayed-acute-subdural-hematoma-treated-with-endoscopic-procedure-a-case-report/
Abstract
Background: Delayed acute subdural hematoma (DASDH) is defined as late onset ASDH with the absence of any abnormal radiological and clinical findings at initial examination. Moreover, this entity is very rare in traumatic brain injury and its mechanism is still unclear. Recently, endoscopic surgery for ASDH has also been performed. In this case, we describe some considerations of the mechanism of DASDH and review previous literature and usefulness of endoscopic surgical procedure for ASDH.
Case Description: A 73-year-old man fell at night, and visited a former medical institution by himself. No abnormal neurological finding was detected. Head computed tomography (CT) detected no abnormal finding. He was diagnosed minor head injury and was hospitalized at midnight and discharged after brain magnetic resonance image (MRI) next day. Brain MRI also detected no abnormal findings. Three days later, he visited our hospital himself, because of the severe headache. Neurologically, he had a mild consciousness disturbance and head CT revealed left ASDH. We performed endoscopic evacuation of hematoma under local anesthesia. Then, the clot was evacuated under the endoscopic procedure through dilated burr hole and pulsatile bleeding from the cortical artery was observed, which was considered to be the source of the ASDH. The patient’s consciousness disturbance was improved immediately after surgery and he discharged without neurological deficit.
Conclusion: We revealed the source of bleeding of DASDH under endoscopic procedure and described hypothesis and speculation of its cause in our case. DASDH is rare entity, so we need further experiences and more considerations.
Keywords: Bleeding source, Delayed acute subdural hematoma, Endoscopic procedure, Mechanism of bleeding
INTRODUCTION
Acute subdural hematoma (ASDH) is well known as a severe prognostic factor of head trauma that requires an emergency surgical treatment.[
We often experienced cases that have increased hematoma during the course and require additional treatment. Moreover, the standard treatment for ASDH is large craniotomy and decompressive craniectomy to prevent secondary brain damage.
Delayed ASDH (DASDH) is very rare concept that defined as late onset ASDH with the absence of any abnormal radiological and clinical findings at initial examination.[
In this report, we detected a cause of DASDH, during endoscopic surgical procedure and describe some considerations of the mechanism of DASDH, surgical procedure, and review previous literature.
CASE REPORT
A 73-year-old man fell while walking to get to the bathroom late at night and was injured his head, and visited a former medical institution by himself.
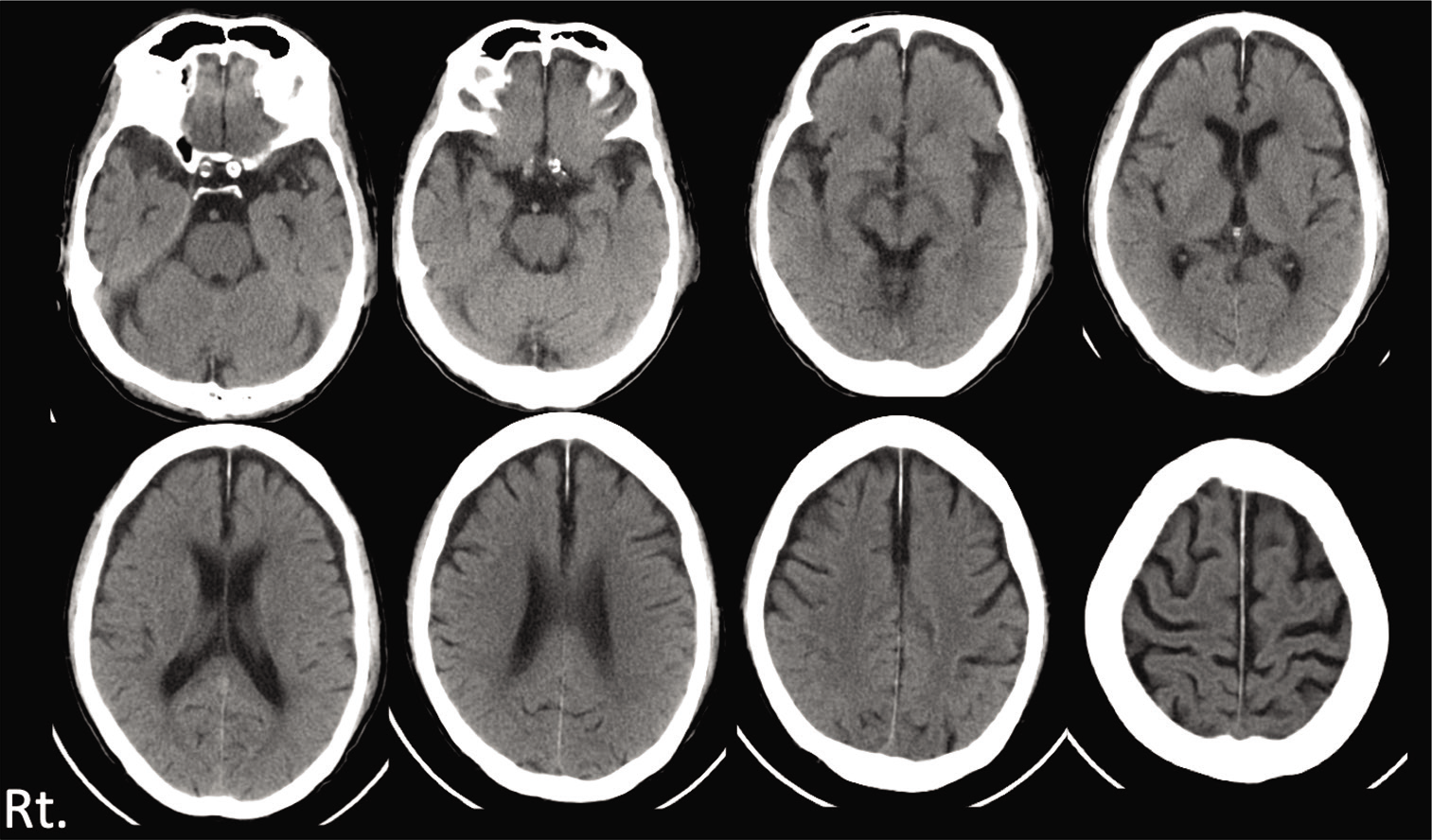
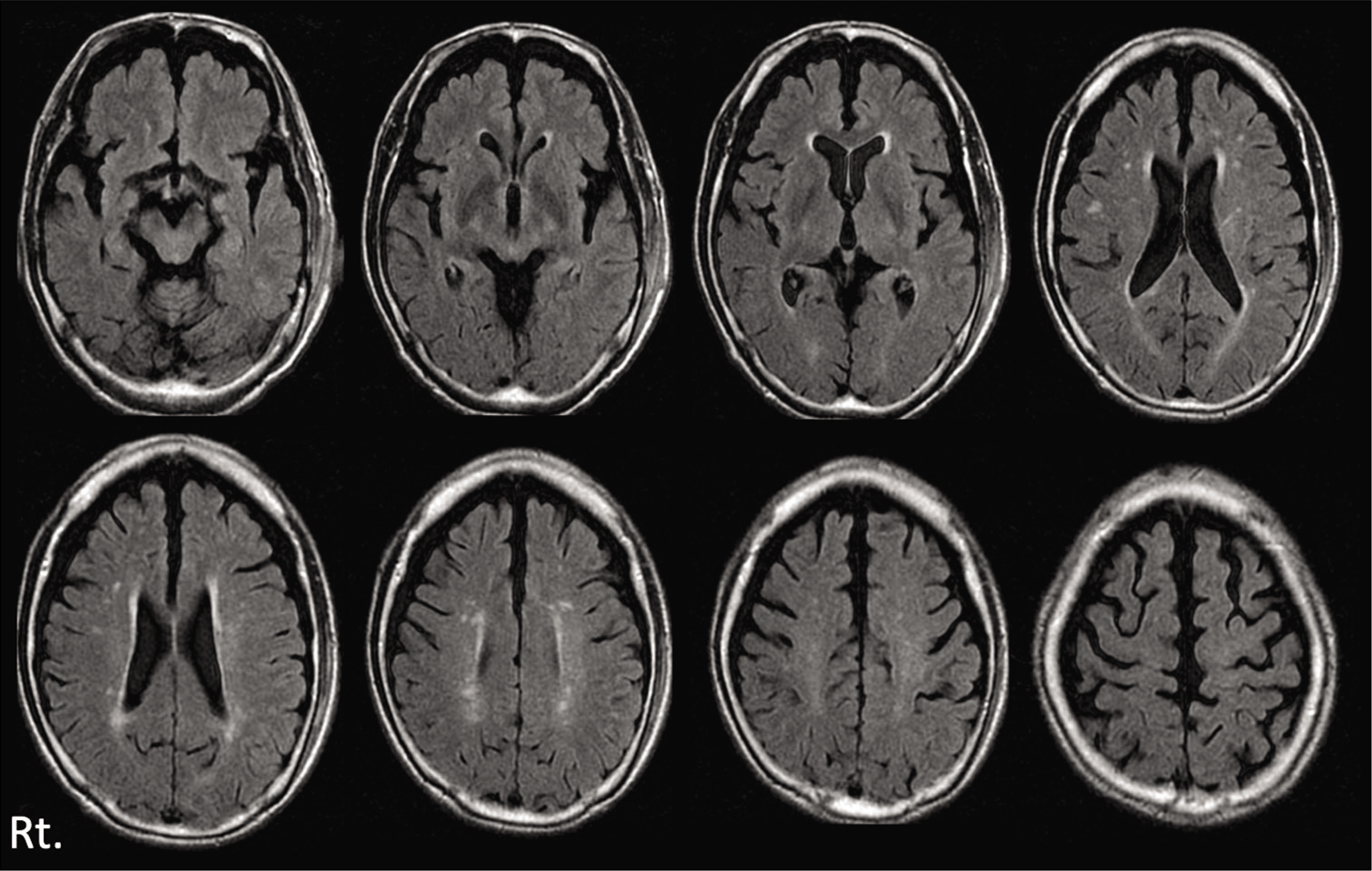
His consciousness was clear (Glasgow Coma Scale: GCS E4V5M6) and there were no abnormal neurological findings including loss of consciousness or amnesia. Head computed tomography (CT) was performed and detected no abnormal finding [
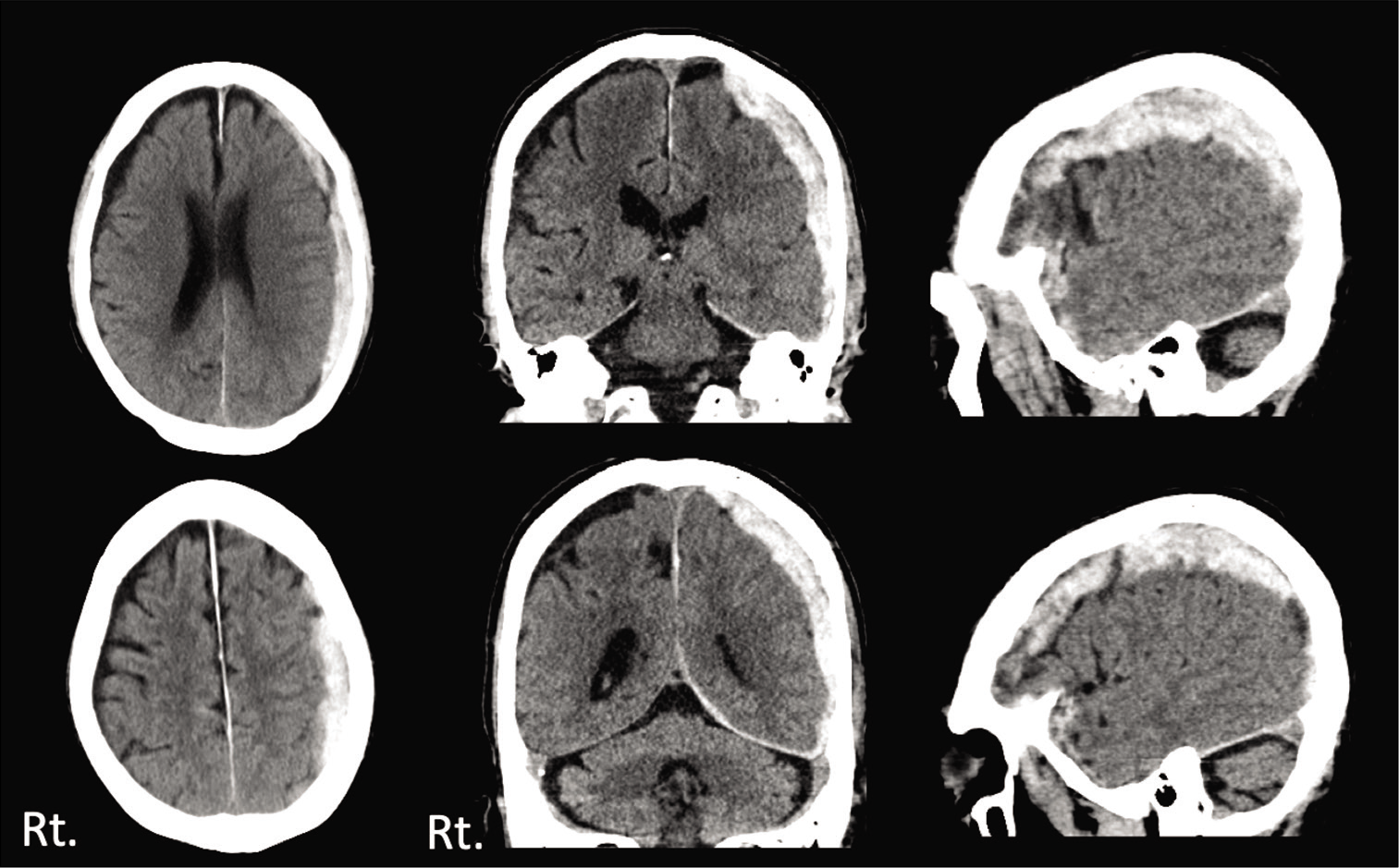
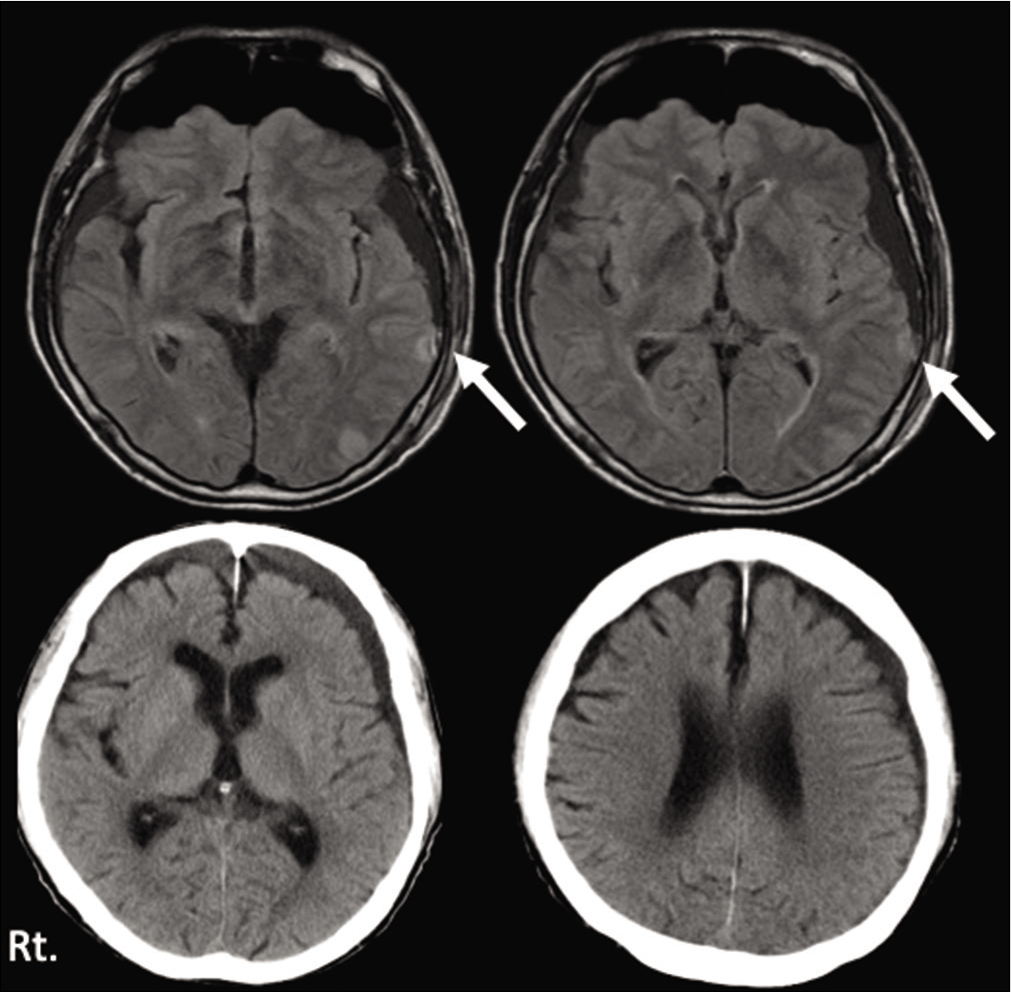
Three days later, he visited our hospital himself, because of the severe headache. Neurologically, he had a mild consciousness disturbance (GCS E4V4M6) and head CT revealed left ASDH with mass effect [
DISCUSSION
There is an approximately 1% risk of life-threatening, intracranial hemorrhagic events that needs immediate neurosurgical operation both in adults and children of mild traumatic brain injury (mTBI).[
Patients have risk factors, such as coagulation disorder, taking anticoagulants or antiplatelets, alcoholism, deranged liver function, and chronic illnesses, such as diabetes mellitus, hypertension, and underlying cardiac disease. Especially elderly patients, increasing oral antiplatelet or anticoagulation have been shown to be associated with higher risk of intracranial bleeding after head injury.
Especially elderly patients, increasing oral antiplatelet or anticoagulation have been shown to be associated with higher risk of intracranial bleeding after head injury.
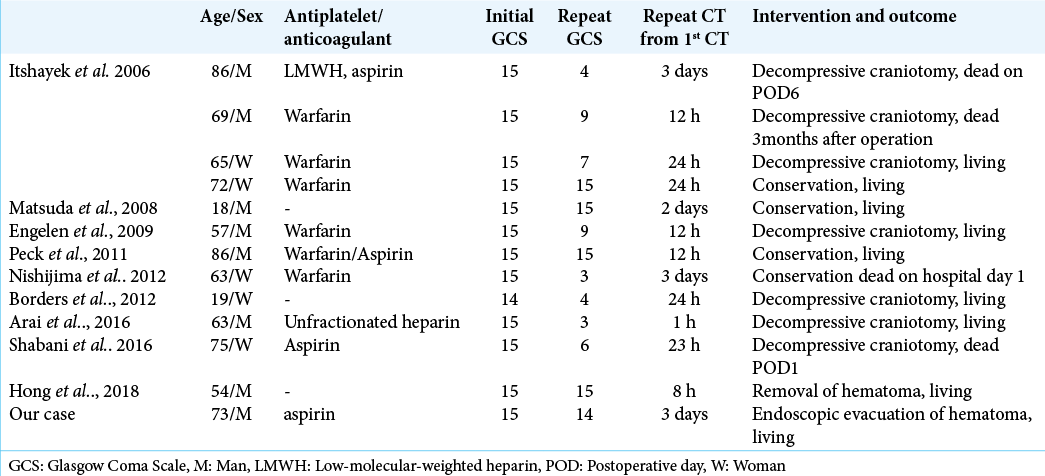
In our review, there are 13 cases (including our case) of DASDH after posttraumatic injury [
Moreover, these nine patients were administered antiplatelet and/or anticoagulant therapy [
In all cases were diagnosed mTBI which GCS 14–15 at the initial evaluation. And their neurological symptoms were deteriorated between 3 and 72 h in our review. When the symptoms worsen, the GCS score is 8 or less, and the prognosis is very poor [
The mechanism of DASDH is still unclear. As described by Zervas et al., the cerebral blood vessels are devoid of vasa vasorum.[
In our case, it was presumed that slight damage to the vessel wall occurred first, and then delayed disruption of the vessel occurred due to failure of rete vasorum. We speculated that the antiplatelet might have contributed to delayed bleeding. To confirm this phenomenon, we need to accumulate more cases and further evaluation. Arterial or venous bleeding as a source of ASDH is often seen in craniotomy. However, this is the first report to describe the bleeding point of DASDH and get hemostasis under endoscopic procedure.
Although, the usefulness of endoscopic surgery as a minimally invasive surgery for subdural hematoma in elderly and also young patients have been reported recently,[
The indication and contraindication of endoscopic surgery for SDH have been reported.[
The development of useful equipment is important. When making a burr hole as an endoscopic surgical corridor for intracranial hemorrhagic lesions, such as a subdural hematoma, it is necessary to make one with enough size. We developed a novel instrument in the form of a dilator attachment with a cordless handle, making it simple, and safe to extend a burr hole after perforation.[
Yokosuka created an irrigation suction cannula with a malleable nozzle to improve procedures in the subdural space.[
Fortunately, our case had no remarkable brain swelling or massive bleeding during surgery; we were able to accomplish the endoscopic procedure with no trouble.
There are no reports that have been made regarding the source of bleeding of DASDH, and it is difficult to explain the mechanism clearly in this case, but this is the first report in which this case was clearly confirmed the source of DASDH during surgery with especially endoscopic procedure.
CONCLUSION
We report a case of DASDH and hypothesis of its pathogenesis based on findings of endoscopic surgical procedure. DASDH is rare entity, so we need further experiences and more considerations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Arai N, Nakamura A, Tabuse M, Miyazaki H. Delayed acute subdural hematoma associated with percutaneous coronary intervention. J Cranniofac Surg. 2016. 27: 514-6
2. Bordes J, Goutorbe P, Lacroix G, Prunet B, Asencio Y, Kaiser E. A case of massive delayed acute subdural hematoma. J Emerg Med. 2012. 42: 459-60
3. Codd PJ, Venteicher AS, Agarwalla PK, Kahle KT, Jho DH. Endoscopic burr hole evacuation of an acute subdural hematoma. J Clin Neurosci. 2013. 20: 1751-3
4. Diaz FG, Yock DH, Larson D, Rockswold GL. Early diagnosis of delayed posttraumatic intracerebral hematomas. J Neurosurg. 1979. 50: 217-23
5. Engelen M, Nederkoorn PJ, Smits M, Van De Beek D. Delayed life-threatening subdural hematoma after minor head injury in a patient with severe coagulopathy: A case report. Cases J. 2009. 2: 7587
6. Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir (Wien). 1998. 90: 111-6
7. Hong SO, Kang DS, Kong MH, Jang SY, Kim JH, Song KY. Development of delayed acute subdural hematoma after mild traumatic brain injury: A case report. Korean J Neurotrauma. 2018. 14: 24-7
8. Itshayek E, Rosenthal G, Fraifeld S, Perez-Sanchez X, Cohen JE, Spektor S. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006. 58: 851-6
9. Karakhan VB, Khodnevich AA. Endoscopic surgery of traumatic intracranial haemorrhages. Acta Neurochir Suppl. 1994. 61: 84-91
10. Kon H, Saito A, Uchida H, Inoue M, Sasaki T, Nishijima M. Endoscopic surgery for traumatic acute subdural hematoma. Case Rep Neurol. 2013. 5: 208-13
11. Kuge A, Kondo R, Sato S, Mitobe Y, Saito S, Sonoda Y. Novel burr hole dilator for endoscopic surgery for intracranial hemorrhagic lesion: Technical note. World Neurosurg. 2019. 128: 295-8
12. Kuge A, Tsuchiya D, Watanabe S, Sato M, Kinjo T. Endoscopic hematoma evacuation for acute subdural hematoma in a young patient: A case report. Acute Med Surg. 2017. 4: 451-3
13. Matsuda W, Sugimoto K, Sato N, Watanabe T, Fujimoto A, Matsumura A. Delayed onset of posttraumatic acute subdural hematoma after mild head injury with normal computed tomography: A case report and brief review. J Trauma. 2008. 65: 461-3
14. Miki K, Yoshioka T, Hirata Y, Enomoto T, Takagi T, Tsugu H. Surgical outcome of acute and subacute subdural hematoma with endoscopic surgery. No Shinkei Geka. 2016. 44: 455-62
15. Nishijima DK, Offerman SR, Ballard DW, Vinson DR, Chettipally UK, Rauchwerger AS. Immediate and delayed traumatic intracranial hemorrhage in patients with head trauma and preinjury warfarin or clopidogrel use. Ann Emerg Med. 2012. 59: 460-8
16. Peck KA, Sise CB, Shackford SR, Sise MJ, Calvo RY, Sak DI. Delayed intracranial hemorrhage after blunt trauma: Are patients on preinjury anticoagulants and prescription antiplatelet agents at risk?. J Trauma. 2011. 71: 600-4
17. Shabani S, Nguyen HS, Doan N, Baisden JL. Case report and review of literature of delayed acute subdural hematoma. World Neurosurg. 2016. 96: 66-71
18. Vos PE, Alekseenko Y, Battistin L, Ehler E, Gerstenbrand F, Muresanu DF. Mild traumatic brain injury. Eur J Neurol. 2012. 19: 191-8
19. Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: Morbidity, mortality, and operative timing. J. Neurosurg. 1991. 74: 212-8
20. Yokosuka K, Uno M, Matsumura K, Takai H, Hagino H, Matsushita N. Endoscopic hematoma evacuation for acute and subacute subdural hematoma in elderly patients. J. Neurosurg. 2015. 123: 1065-9
21. Zervas NT, Liszczak TM, Mayberg MR, Black PM. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg. 1982. 56: 475-81