- Department of Neurosurgery, Iwate Medical University, Yahaba, Japan
- Central Clinical Laboratory, Iwate Medical University, Yahaba, Japan.
Correspondence Address:
Hiroaki Saura, Department of Neurosurgery, Iwate Medical University, Yahaba, Japan.
DOI:10.25259/SNI_422_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sotaro Oshida1, Hiroaki Saura1, Yosuke Akamatsu1, Wataru Yanagihara1, Kentaro Fujimoto1, Kazuki Nagasawa2, Kodai Takahashi2, Kuniaki Ogasawara1. Delayed blink R1 latency in a patient with trigeminal neuralgia due to a contralateral vestibular schwannoma: An illustrative case. 11-Aug-2023;14:284
How to cite this URL: Sotaro Oshida1, Hiroaki Saura1, Yosuke Akamatsu1, Wataru Yanagihara1, Kentaro Fujimoto1, Kazuki Nagasawa2, Kodai Takahashi2, Kuniaki Ogasawara1. Delayed blink R1 latency in a patient with trigeminal neuralgia due to a contralateral vestibular schwannoma: An illustrative case. 11-Aug-2023;14:284. Available from: https://surgicalneurologyint.com/surgicalint-articles/12496/
Abstract
Background: Although the blink reflex (BR) is effective in objectively evaluating trigeminal neuropathy, few studies have demonstrated its effect on trigeminal neuralgia (TN). The authors report a patient with TN due to contralateral vestibular schwannoma (VS) functionally diagnosed by delayed R1 latency of the BR.
Case Description: A 36-year-old man presented with left-sided deafness and paroxysmal facial pain in the right V1-3 area. Magnetic resonance imaging (MRI) showed a solid cystic mass compressing the right pons and left brainstem at the left cerebellopontine angle. Although preoperative BR evoked by right supraorbital nerve stimulation-induced delayed ipsilateral R1 latency and normal ipsilateral and contralateral R2 responses, the BR latency evoked by left supraorbital nerve stimulation was normal, indicating deficits in the principal nucleus of the trigeminal nerve in the right pons. The symptoms of TN disappeared after the removal of the VS. Postoperative MRI showed subtotal removal of the tumor and sufficient decompression of the pons and cerebellopontine cistern. The R1 latency returned to normal 50 days after surgery.
Conclusion: The perioperative BR test was not only useful for objective evaluation of the localization of trigeminal neuropathy but also correlated with the symptoms of TN.
Keywords: Blink reflex, Contralateral vestibular schwannoma, Trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) can occur due to compression of the root entry zone (REZ) of the trigeminal nerve.[
CASE DESCRIPTION
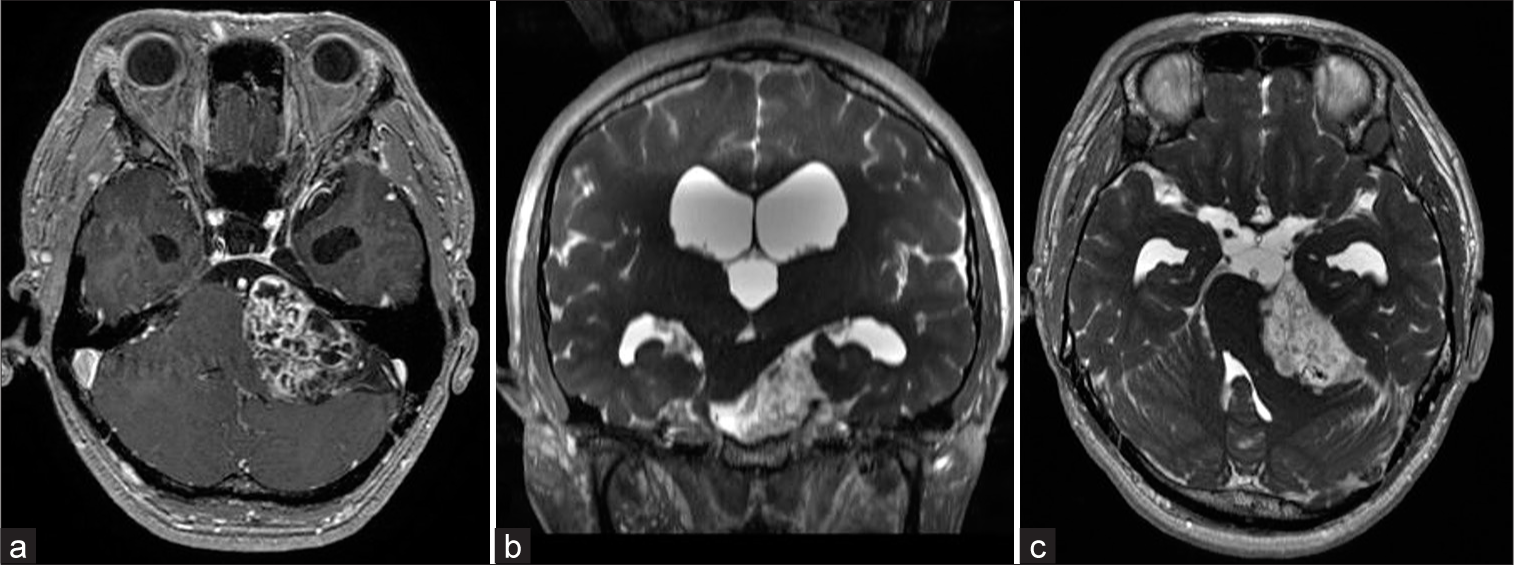
A 36-year-old man with an unremarkable medical history experienced recurrent right facial pain in the second and third division of the trigeminal nerve for 27 months. Facial pain was triggered by chewing and tooth brushing, which occurred about 6 times a day with each episode lasting for 10 min. Nine months after the initial onset, facial pain spread to the first division of the trigeminal nerve. Carbamazepine administration slightly alleviated the pain but did not eliminate it; therefore, the patient was referred to our department for further evaluation and treatment. Neurological examination revealed left-sided deafness, left Bruns’ nystagmus, bilateral papilledema, and paroxysmal electric pain in all right trigeminal nerve divisions. Magnetic resonance imaging (MRI) showed a heterogeneously enhanced mass measuring 4.8 × 3.3 cm in the left cerebellopontine angle (CPA) and a tight cerebellopontine cistern of the right CPA [
Figure 1:
Preoperative axial T1-weighted enhanced magnetic resonance imaging showing a well-margined, heterogeneous enhancing mass at the left cerebellopontine angle (48 × 33 × 34 mm), compatible with a vestibular schwannoma (a). Preoperative coronal fast imaging employing steady-state acquisition (FIESTA) showed that the brainstem was compressed by the tumor and deviated contralaterally (b). Preoperative axial FIESTA revealed a tight cerebellopontine cistern on the right side, and the trigeminal nerve was deviated and compressed toward the petrous bone. There were no obvious vessels responsible for the compression of the right trigeminal nerve, such as the superior cerebellar artery (c).
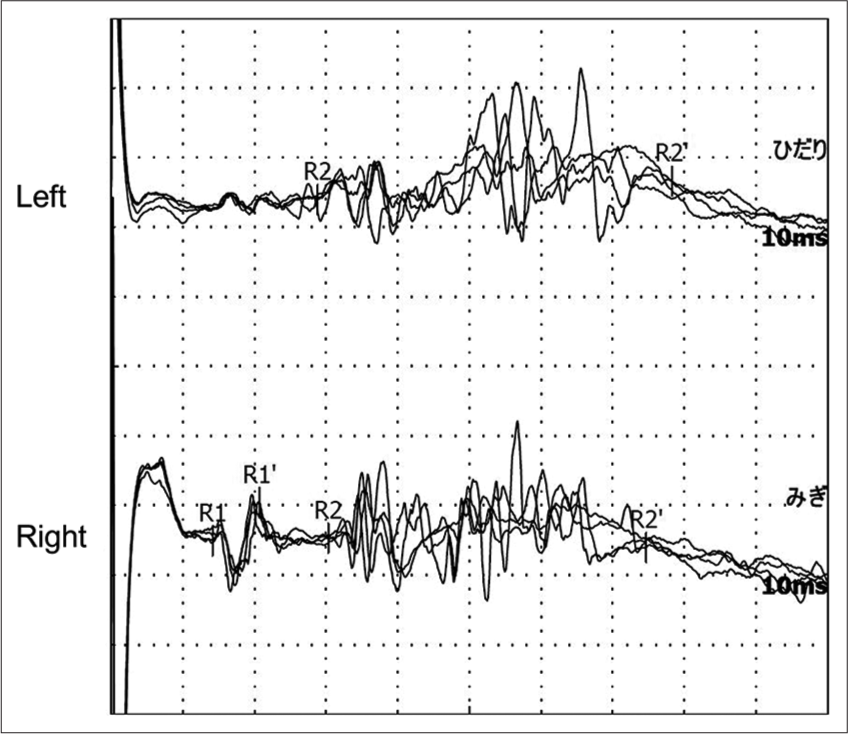
For further evaluation of right facial pain, the bilateral BR was obtained using bipolar stimulation by placing the cathode in the supraorbital crease and the anode 2 cm above the cathode. Recording electrodes were placed on the lower eyelids, with a reference electrode near each lateral cantus. Stimulation with 0.2 ms square-wave pulses was measured using Neuropack X1 (Nihon Kohden, Japan). The latency of the R1 and R2 waves ipsilateral and contralateral to the stimulus and the difference between the R1 latency ipsilateral and contralateral to the lesion were measured. We referred to previous study indices of normal neurophysiology values expressed as the mean ± SD in ms: R1, 10.5 ± 1.6; R2 ipsilateral, 30.5 ± 6.4; R2 contralateral, 30.5 ± 3.4; the difference between right and left R1, 1.2; and mean upper limit, +2 SD.[
Figure 2:
Blink reflex with a lesion elicited with right supraorbital nerve stimulation. Reflex responses were recorded on the left (upper) and right (lower) eyelids. Stimulation of the right supraorbital nerve elicited an ipsilateral R1 response (lower) with a delayed latency of 14.2 ms, an ipsilateral R2 response with a normal latency of 30.3 ms, and contralateral R2 response (upper) with a normal latency of 28.8 ms.
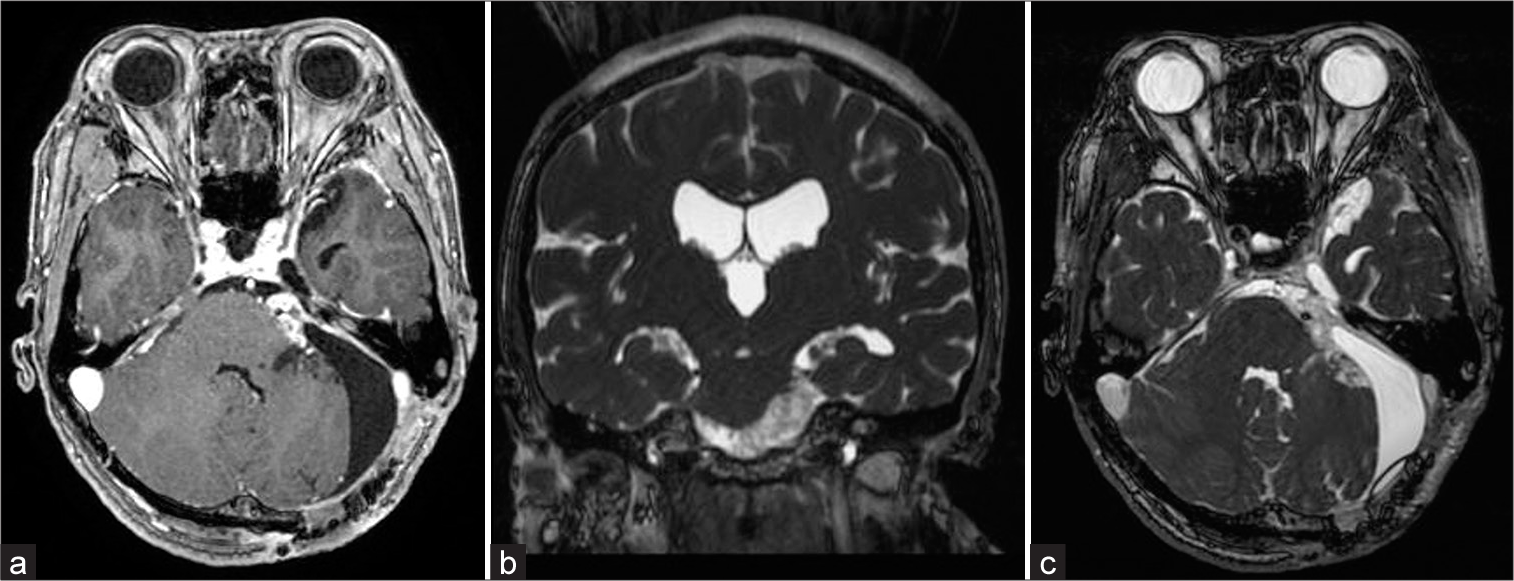
The tumor was removed using the left retrosigmoid approach under general anesthesia with auditory brainstem response (ABR), motor-evoked potential (MEP), and sensory-evoked potential monitoring. The MEP was used to monitor brainstem injury. During surgery, the preservation of facial nerve function was confirmed using a neural integrity monitor. The ABR and MEP results did not change postoperatively. Finally, complete tumor resection was performed, while avoiding facial nerve and brainstem dysfunction. The tumor was diagnosed as a grade 1 schwannoma based on histopathological examination (the World Health Organization criteria). Because his right facial pain disappeared 5 days after surgery, carbamazepine was gradually tapered off until completely discontinued on the 45th postoperative day. Postoperative MRI demonstrated a significant reduction in brainstem compression and widening of the right cerebellopontine cistern [
Figure 3:
Postoperative T1-weighted enhanced magnetic resonance (MR) imaging showed a small residual tumor and enlargement of the cerebellopontine cistern (a). Postoperative coronal fast imaging employing steady-state acquisition (FIESTA) MR images showed that the brainstem deviation was improved (b). Postoperative axial FIESTA MR images showed normalization of the right cerebellopontine cistern, normalization of the brainstem, and trigeminal nerve position compared to before tumor resection (c).
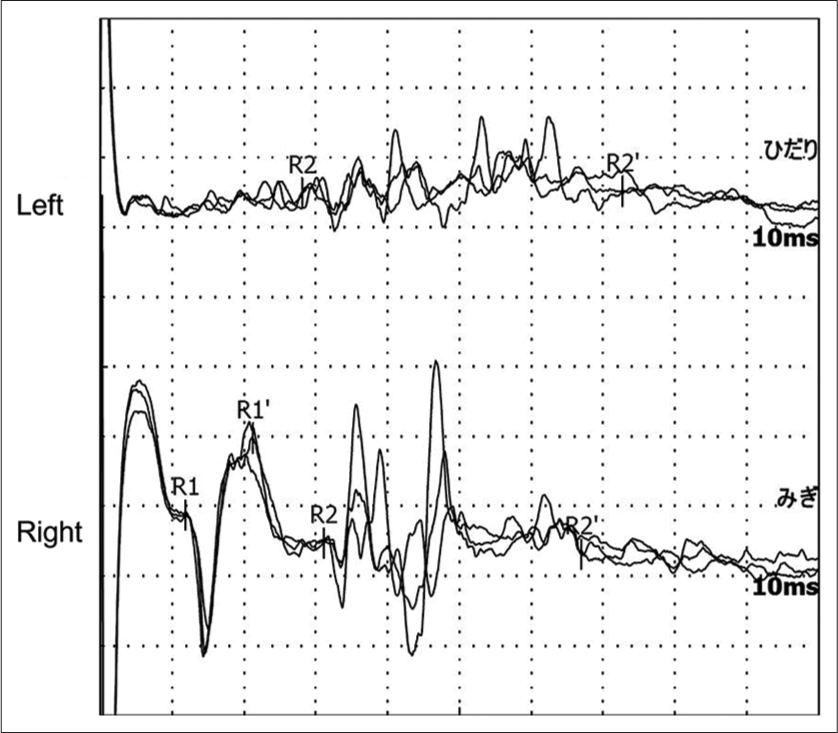
Figure 4:
Blink reflex elicited with a lesion on the right supraorbital nerve. Reflex responses were recorded on the lower left (upper) and right (lower) eyelids. Stimulation of the right supraorbital nerve elicited an ipsilateral R1 response (lower) with a normal latency of 11.9 ms, an ipsilateral R2 response with a normal latency of 31.1 ms, and contralateral R2 response (upper) with a normal latency of 28.1 ms.
DISCUSSION
BR testing has been performed to evaluate the function of the trigeminal and facial nerves, which elicit two distinct responses to supraorbital nerve stimulation: R1 and R2 waves.[
CONCLUSION
Pre- and postoperative BR testing might be a feasible modality to objectively evaluate the localization of the TN and perioperative changes in trigeminal nerve function, such as neuralgia.
Disclosures
The authors have no personal, financial, or institutional interests in any of the drugs, materials, or devices described in this article.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aramideh M, Ongerboer de Visser BW, Koelman JH, Majoie CB, Holstege G. The late blink reflex response abnormality due to lesion of the lateral tegmental field. Brain. 1997. 120: 1685-92
2. Berra LV, Armocida D, Mastino L, Rita AD, Norcia VD, Santoro A. Trigeminal neuralgia secondary to intracranial neoplastic lesions: A case series and comprehensive review. J Neurol Surg A Cent Eur Neurosurg. 2021. 82: 118-24
3. Cenzato M, Stefini R, Ambrosi C, Latronico N, Milani D. Surgical resolution of trigeminal neuralgia due to intra-axial compression by pontine cavernous angioma. World Neurosurg. 2010. 74: 544-6
4. Chamadoira C, Cerejo A, Duarte F, Vaz R. Trigeminal neuralgia caused by contra lateral cerebellopontine angle tumor. A case report. Neurocirugia (Astur). 2010. 21: 50-2
5. Eftekhar B, Gheini M, Ghodsi M, Ketabchi E. Vestibular schwannoma with contralateral facial pain-case report. BMC Neurol. 2003. 3: 2
6. Hasegawa T, Kato T, Naito T, Tanei T, Ishii K, Tsukamoto E. Predictors of long-term tumor control after stereotactic radiosurgery for Koos grade 4 vestibular schwannomas. J Neurooncol. 2021. 151: 145-56
7. Hopf HC, Thomke F, Gutmann L. Midbrain vs. pontine medial longitudinal fasciculus lesions: The utilization of masseter and blink reflexes. Muscle Nerve. 1991. 14: 326-30
8. Kugelberg E. Facial reflexes. Brain. 1952. 75: 385-96
9. Kumura J, editors. Electrodiagnosis in Disease of Nerve and Muscle: Principles and Practice. New York: Oxford University Press; 2001. p. 409-38
10. Lefranc M, Da Roz LM, Balossier A, Thomassin JM, Roche PH, Regis J. Place of gamma knife stereotactic radiosurgery in grade 4 vestibular schwannoma based on case series of 86 patients with long-term follow-up. World Neurosurg. 2018. 114: e1192-8
11. Nurlu G, Bavbek M, Colak A, Sarjbaş O, Ozgen T. Brainstem auditory evoked potentials and blink reflexes in patients with pontocerebellar angle tumors. Neurosurg Rev. 1994. 17: 253-60
12. Ongerboer de Visser BW, Cruccu G, Brown WF, Bolton CF, editors. Neurophysiologic examination of the trigeminal, facial, hypoglossal, and spinal accessory nerves in cranial neuropathies and brain stem disorders. Clinical Electromyography. Boston: Butterworth-Heinemann; 1993. p. 61-92
13. Paillas JE, Pellet W, Janny P, Tournilhac M, Komminoth J. Controlateral involvement of cranial nerves in posterior fossa tumors. Rev Neurol (Paris). 1969. 121: 452-64
14. Parker HL. Paroxysmal trigeminal pain with tumours of the nervus acusticus. J Neurol Psychopathol. 1937. 17: 256-61
15. Samii M, Matthies C. Acoustic neurinomas associated with vascular compression syndromes. Acta Neurochir (Wien). 1995. 134: 148-54
16. Seo Y, Kim DG. Contralateral trigeminal neuralgia in a rapidly growing vestibular schwannoma: A case report. J Clin Neurosci. 2018. 47: 132-4
17. Snow RB, Fraser RA. Cerebellopontine angle tumor causing contralateral trigeminal neuralgia: A case report. Neurosurgery. 1987. 21: 84-6
18. Tanaka A, Takaki T, Maruta Y. Neurinoma of the trigeminal root presenting as atypical trigeminal neuralgia: Diagnostic values of orbicularis oculi reflex and magnetic resonance imaging. A case report. Neurosurgery. 1987. 21: 733-6